��Ŀ����
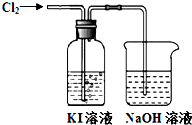 ijͬѧ��Cl2��KI��Һ�ķ�Ӧ������ʵ��̽������Ӧװ�����£�
ijͬѧ��Cl2��KI��Һ�ķ�Ӧ������ʵ��̽������Ӧװ�����£�ͨ������һ��ʱ�䣬KI��Һ��Ϊ��ɫ������ͨ������һ��ʱ�����Һ��ɫ��ȥ����Ϊ��ɫ������ͨ�������������Һ��Ϊdz����ɫ��
��1��Cl2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��2��KI��Һ��Ϊ��ɫ˵���������е�
��3����֪I2+I- I3-��I2��I3-��ˮ�о��ʻ�ɫ��Ϊȷ����ɫ��Һ�ijɷ֣�����������ʵ�飮
��ʵ��b��Ŀ����
�ڸ���ʵ��a�У�ˮ���к��е�������
| ���� | ʵ������ | |
| a | ȡ2��3 mL��ɫ��Һ����������CCl4�����ã� | CCl4����Ϻ�ɫ��ˮ����dz��ɫ�� |
| b | ȡ2��3 mL���͵�ˮ����������CCl4�����ã� | CCl4����Ϻ�ɫ��ˮ�㼸����ɫ�� |
��Ϊ��֤ʵ����Ͻ��ԣ���ʵ��a��b�Ļ����ϣ��貹��һ��ʵ�飬��ʵ��Ϊ
��4��ͨ����������Һ�ɻ�ɫ��Ϊ��ɫ������Ϊ������I2��������֪1mol Cl2������0.2mol I2���÷�Ӧ�Ļ�ѧ����ʽ��
��5����������ʵ�飬��Ԥ�������-KI��Һ�г���ͨ�����������ܹ۲쵽������Ϊ
��6����Һ���ձ�Ϊdz��ɫ��ԭ����
���㣺����ʵ�鷽�������
ר�⣺
��������1���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��2��ͨ��������KI��Һ��Ϊ��ɫ��˵�����ɵⵥ�ʣ�˵�������������Աȵ�ǿ��
��3��������KI����Cl2+2I-=I2+2Cl-���Լ�I2+I-?I3-��ȡ2��3mL��ɫ��Һ����������CCl4�����ã�CCl4����Ϻ�ɫ��ˮ����dz��ɫ����˵����Һ�д���I2��I3-��
��4��ͨ����������Һ�ɻ�ɫ��Ϊ��ɫ������Ϊ������I2������1mol Cl2������0.2mol I2��˵��IԪ�ػ��ϼ�����5�ۣ�Ӧ����HIO3��
��5�������-KI��Һ�г���ͨ�������������ɵ��ʵ⣬��Һ�����ɫ������ͨ���������ⱻ��������HIO3����Һ��ɫ��
��6����ˮ����ɫΪdz��ɫ��
��2��ͨ��������KI��Һ��Ϊ��ɫ��˵�����ɵⵥ�ʣ�˵�������������Աȵ�ǿ��
��3��������KI����Cl2+2I-=I2+2Cl-���Լ�I2+I-?I3-��ȡ2��3mL��ɫ��Һ����������CCl4�����ã�CCl4����Ϻ�ɫ��ˮ����dz��ɫ����˵����Һ�д���I2��I3-��
��4��ͨ����������Һ�ɻ�ɫ��Ϊ��ɫ������Ϊ������I2������1mol Cl2������0.2mol I2��˵��IԪ�ػ��ϼ�����5�ۣ�Ӧ����HIO3��
��5�������-KI��Һ�г���ͨ�������������ɵ��ʵ⣬��Һ�����ɫ������ͨ���������ⱻ��������HIO3����Һ��ɫ��
��6����ˮ����ɫΪdz��ɫ��
���
�⣺��1���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+Cl2�TNaCl+NaClO+H2O���ʴ�Ϊ��2NaOH+Cl2�TNaCl+NaClO+H2O��
��2��ͨ��������KI��Һ��Ϊ��ɫ��˵�����ɵⵥ�ʣ�˵�������������Աȵ�ǿ����Ӧ�����ӷ���ʽΪ2I-+Cl2=2Cl-+I2��
�ʴ�Ϊ�������ԣ�2I-+Cl2=2Cl-+I2 ��
��3����ʵ��b������Ա�ʵ�飬��֤��a����Һ����I3-��˵������I2+I-?I3-ƽ�⣬���ǵ�һ��I2Ũ�Ƚ��������µģ�
�ʴ�Ϊ���Ա�ʵ�飬֤��ʵ��a��ˮ����dz��ɫ����Ϊ����I2+I-?I3-ƽ�⣬���ǵ�һ��I2Ũ�Ƚ��������µģ�
�ڴ���I2+I-?I3-ƽ�⣬����Һ�д���I2��I-��I3-���������Cl-��K+���ʴ�Ϊ��I2��I-��I3-��Cl-��K+��
�۴���I2+I-?I3-ƽ�⣬��Һ�д���I2���������Ȼ�̼�������������Ȼ�̼����Һ�е�Ũ�Ƚ��ͣ���I2+I-?I3-ƽ���������ƶ���I3-Ũ��Ҳ���ͣ�����ˮ��Һ��ɫ��dz��
�ʴ�Ϊ��������ȡʹˮ��Һ��I2Ũ�Ƚ��ͣ�ͬʱI2+I-?I3-ƽ���������ƶ���I3-Ũ��Ҳ���ͣ�����ˮ��Һ��ɫ��dz��
��Ϊ��֤ʵ����Ͻ��ԣ���ʵ��a��b�Ļ����ϣ���ȡ2��3 mL KI��Һ���μ���������ˮ����ͨ����������������������ⵥ�ʣ����ټ�������CCl4�����ã��۲쵽ˮ���Ƿ�Ϊ��ɫ����ȡ����ʵ��a��ˮ����Һ�μ�AgNO3��Һ���۲��Ƿ��л�ɫ�������ɣ���ȡ����ʵ��a��ˮ����Һ������Һ���۲��Ƿ������
�ʴ�Ϊ��ȡ2��3 mL KI��Һ���μ���������ˮ����ͨ����������������������ⵥ�ʣ����ټ�������CCl4�����ã��۲쵽ˮ���Ƿ�Ϊ��ɫ��
[��ȡ����ʵ��a��ˮ����Һ�μ�AgNO3��Һ���۲��Ƿ��л�ɫ�������ɣ���ȡ����ʵ��a��ˮ����Һ������Һ���۲��Ƿ����]��
��4��������Cl2��I2��KI��Һ��ͨ����������Cl2+2KI=KCl+I2����Һ��ɻ�ɫ������ͨ������������5Cl2+I2+6H2O=2HIO3+10HCl��
�ʴ�Ϊ��I2+5Cl2+6H2O=10HCl+2HIO3��
��5�������-KI��Һ�г���ͨ�������������ɵ��ʵ⣬��Һ�����ɫ������ͨ���������ⱻ��������HIO3����Һ��ɫ���ʴ�Ϊ����Һ�ȱ�������ɫ��
��6�����������������������ܽ���ˮ��ˮ�к����������ӣ���ˮ��dz��ɫ���ʴ�Ϊ�����������������������ܽ���ˮ��ˮ�к����������ӣ�
��2��ͨ��������KI��Һ��Ϊ��ɫ��˵�����ɵⵥ�ʣ�˵�������������Աȵ�ǿ����Ӧ�����ӷ���ʽΪ2I-+Cl2=2Cl-+I2��
�ʴ�Ϊ�������ԣ�2I-+Cl2=2Cl-+I2 ��
��3����ʵ��b������Ա�ʵ�飬��֤��a����Һ����I3-��˵������I2+I-?I3-ƽ�⣬���ǵ�һ��I2Ũ�Ƚ��������µģ�
�ʴ�Ϊ���Ա�ʵ�飬֤��ʵ��a��ˮ����dz��ɫ����Ϊ����I2+I-?I3-ƽ�⣬���ǵ�һ��I2Ũ�Ƚ��������µģ�
�ڴ���I2+I-?I3-ƽ�⣬����Һ�д���I2��I-��I3-���������Cl-��K+���ʴ�Ϊ��I2��I-��I3-��Cl-��K+��
�۴���I2+I-?I3-ƽ�⣬��Һ�д���I2���������Ȼ�̼�������������Ȼ�̼����Һ�е�Ũ�Ƚ��ͣ���I2+I-?I3-ƽ���������ƶ���I3-Ũ��Ҳ���ͣ�����ˮ��Һ��ɫ��dz��
�ʴ�Ϊ��������ȡʹˮ��Һ��I2Ũ�Ƚ��ͣ�ͬʱI2+I-?I3-ƽ���������ƶ���I3-Ũ��Ҳ���ͣ�����ˮ��Һ��ɫ��dz��
��Ϊ��֤ʵ����Ͻ��ԣ���ʵ��a��b�Ļ����ϣ���ȡ2��3 mL KI��Һ���μ���������ˮ����ͨ����������������������ⵥ�ʣ����ټ�������CCl4�����ã��۲쵽ˮ���Ƿ�Ϊ��ɫ����ȡ����ʵ��a��ˮ����Һ�μ�AgNO3��Һ���۲��Ƿ��л�ɫ�������ɣ���ȡ����ʵ��a��ˮ����Һ������Һ���۲��Ƿ������
�ʴ�Ϊ��ȡ2��3 mL KI��Һ���μ���������ˮ����ͨ����������������������ⵥ�ʣ����ټ�������CCl4�����ã��۲쵽ˮ���Ƿ�Ϊ��ɫ��
[��ȡ����ʵ��a��ˮ����Һ�μ�AgNO3��Һ���۲��Ƿ��л�ɫ�������ɣ���ȡ����ʵ��a��ˮ����Һ������Һ���۲��Ƿ����]��
��4��������Cl2��I2��KI��Һ��ͨ����������Cl2+2KI=KCl+I2����Һ��ɻ�ɫ������ͨ������������5Cl2+I2+6H2O=2HIO3+10HCl��
�ʴ�Ϊ��I2+5Cl2+6H2O=10HCl+2HIO3��
��5�������-KI��Һ�г���ͨ�������������ɵ��ʵ⣬��Һ�����ɫ������ͨ���������ⱻ��������HIO3����Һ��ɫ���ʴ�Ϊ����Һ�ȱ�������ɫ��
��6�����������������������ܽ���ˮ��ˮ�к����������ӣ���ˮ��dz��ɫ���ʴ�Ϊ�����������������������ܽ���ˮ��ˮ�к����������ӣ�
���������⿼��������ⷴӦ�����ʣ�Ϊ��Ƶ���㣬������ѧ���ķ�������ʵ�������Ŀ��飬�ѶȲ���ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
��0.2mol?L-1HCN��Һ��0.1mol?L-1��NaOH��Һ�������Ϻ���Һ�Լ��ԣ����й�ϵʽ����ȷ���ǣ�������
| A��c ��HCN����c ��CN-�� |
| B��c ��Na+����c ��CN-�� |
| C��c ��HCN��-c ��CN-��=c ��OH-�� |
| D��c ��HCN��+c ��CN-��=0.1mol?L-1 |
���и�˵���У���ȷ���ǣ�������
| A����H��0��ʾ���ȷ�Ӧ����H��0��ʾ���ȷ�Ӧ |
| B���Ȼ�ѧ����ʽ�еĻ�ѧ������ֻ��ʾ���ʵ����������Ƿ��� |
| C��1mol H2SO4��1mol Ba��OH��2��Ӧ����BaSO4����ʱ�ų����Ƚ����к��� |
| D��1mol H2��0.5molO2��Ӧ�ų����Ⱦ���H2��ȼ���� |
25��ijŨ�ȵ����ᡢ�Ȼ����Һ��ˮ�������������Ũ�ȷֱ�Ϊ1.0��10-amol?L-1��1.0��10-bmol?L-1������������Һ��pH֮��Ϊ��������
| A��14-a+b |
| B��14+a+b |
| C��14-a-b |
| D��14+a-b |
 �ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ�� ���黯��Ϊ�������뵼�壬����Ϊ��������ĵ����������������٣���֪�黯�صľ����ṹ��ͼ1��ʾ����ش��������⣺
���黯��Ϊ�������뵼�壬����Ϊ��������ĵ����������������٣���֪�黯�صľ����ṹ��ͼ1��ʾ����ش��������⣺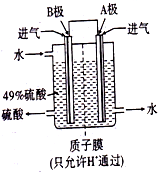 ������ʡ���¼�����������Ӧ�ò��Ϲ�˾��������ҵ�������ҵ�����˲�ҵ�У��趼��������Ҫ�����ã���ش��������⣺
������ʡ���¼�����������Ӧ�ò��Ϲ�˾��������ҵ�������ҵ�����˲�ҵ�У��趼��������Ҫ�����ã���ش��������⣺