��Ŀ����
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף�
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��
�۵ڶ��ݼ�����BaCl2��Һ���ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65g��
��ش��������⣺
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ��
��2��д��Al��OH��3����ǿ������ӷ���ʽ��
ͨ������ʵ�������
��3��һ�������ڵ������� ��
��4������ȷ���Ƿ���ڵ������� ��
��5��ͨ��������ȷ��K+�Ƿ���� ����ǡ���������K+�ķ�����
��6����д���ڲ���Ӧ�����ɰ�ɫ������Ӧ�����ӷ���ʽ��
��7����д���۲���Ӧ�����ӷ���ʽ�� ��
�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף�
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��
�۵ڶ��ݼ�����BaCl2��Һ���ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65g��
��ش��������⣺
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ��
��2��д��Al��OH��3����ǿ������ӷ���ʽ��
ͨ������ʵ�������
��3��һ�������ڵ�������
��4������ȷ���Ƿ���ڵ�������
��5��ͨ��������ȷ��K+�Ƿ����
��6����д���ڲ���Ӧ�����ɰ�ɫ������Ӧ�����ӷ���ʽ��
��7����д���۲���Ӧ�����ӷ���ʽ��
���㣺���������ӵļ���,���������ӵļ���
ר�⣺
�������ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ǰ�����һ����笠����ӣ��������ɣ�һ������Fe3+��Mg2+��
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ�������ó������������������������ˡ�ϴ�ӡ����գ��õ�������������������Ϊ1.02g����0.01mol��������Ԫ���غ㣬���������ӵ����ʵ�����0.02mol��һ������CO32-��
�۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ�����������ᱵ��������������������ϴ�ӡ����������Ϊ11.65g������һ����SO42�����ʵ�����
=0.05mol��һ������Ba2+��������Һ�еĵ���غ�ȷ���������Ƿ���ڣ�
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ�������ó������������������������ˡ�ϴ�ӡ����գ��õ�������������������Ϊ1.02g����0.01mol��������Ԫ���غ㣬���������ӵ����ʵ�����0.02mol��һ������CO32-��
�۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ�����������ᱵ��������������������ϴ�ӡ����������Ϊ11.65g������һ����SO42�����ʵ�����
| 11.65g |
| 233g/mol |
���
�⣺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ǰ�����һ����笠����ӣ�笠����ӵ����ʵ�����0.02mol���������ɣ�һ������Fe3+��Mg2+��
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ�������ó������������������������ˡ�ϴ�ӡ����գ��õ�������������������Ϊ1.02g����0.01mol��������Ԫ���غ㣬���������ӵ����ʵ�����0.02mol��һ������CO32-��
�۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ�����������ᱵ��������������������ϴ�ӡ����������Ϊ11.65g������һ����SO42-�����ʵ�����0.05mol��һ������Ba2+��n��NH4+��+3n��Al3+��=0.02mol+3��0.02mol=0.08mol��2n��SO42-��=0.1mol�����ݵ���غ㣬һ�����ڼ����ӣ���Ϊ��ȷ������Cl-�������ӵ���С���ʵ�����0.02mol��
���ݷ�����֪��һ�������ڵ������У�Fe3+��Mg2+��CO32-��Ba2+��һ�����ڵ������У�K+��SO42-��NH4+��Al3+������ȷ������Cl-��
��1��ʵ���Ҳ�ȡ������ʯ�����Ȼ�淋Ļ�Ϲ�����ȡ��������ѧ����ʽΪ��2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3�����ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2H2O+2NH3����
��2��Al��OH��3����ǿ������ƫ�����κ�ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
ͨ������ʵ�������
��3�����ݷ�����һ�������ڵ������ǣ�Fe3+��Mg2+��CO32-��Ba2+���ʴ�Ϊ��Fe3+��Mg2+��CO32-��Ba2+��
��4������ȷ���Ƿ���ڵ������ǣ�Cl-���ʴ�Ϊ��Cl-��
��5�����ݵ���غ�ó�һ�����ڼأ�������ɫ��Ӧ����֤�����Ƿ���ڣ��ʴ�Ϊ���ǣ���ɫ��Ӧ��
��6��ƫ�������̼�ᷴӦ���������������������ӷ�Ӧ����ʽΪ��AlO2-+CO2+2H2O=Al��OH��3+HCO3-���ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3+HCO3-��
��7�����������������Ӧ�������ᱵ�����ӷ���ʽ��Ba2++SO42-=BaSO4�����ʴ�Ϊ��Ba2++SO42-=BaSO4����
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ�������ó������������������������ˡ�ϴ�ӡ����գ��õ�������������������Ϊ1.02g����0.01mol��������Ԫ���غ㣬���������ӵ����ʵ�����0.02mol��һ������CO32-��
�۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ�����������ᱵ��������������������ϴ�ӡ����������Ϊ11.65g������һ����SO42-�����ʵ�����0.05mol��һ������Ba2+��n��NH4+��+3n��Al3+��=0.02mol+3��0.02mol=0.08mol��2n��SO42-��=0.1mol�����ݵ���غ㣬һ�����ڼ����ӣ���Ϊ��ȷ������Cl-�������ӵ���С���ʵ�����0.02mol��
���ݷ�����֪��һ�������ڵ������У�Fe3+��Mg2+��CO32-��Ba2+��һ�����ڵ������У�K+��SO42-��NH4+��Al3+������ȷ������Cl-��
��1��ʵ���Ҳ�ȡ������ʯ�����Ȼ�淋Ļ�Ϲ�����ȡ��������ѧ����ʽΪ��2NH4Cl+Ca��OH��2
| ||
| ||
��2��Al��OH��3����ǿ������ƫ�����κ�ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
ͨ������ʵ�������
��3�����ݷ�����һ�������ڵ������ǣ�Fe3+��Mg2+��CO32-��Ba2+���ʴ�Ϊ��Fe3+��Mg2+��CO32-��Ba2+��
��4������ȷ���Ƿ���ڵ������ǣ�Cl-���ʴ�Ϊ��Cl-��
��5�����ݵ���غ�ó�һ�����ڼأ�������ɫ��Ӧ����֤�����Ƿ���ڣ��ʴ�Ϊ���ǣ���ɫ��Ӧ��
��6��ƫ�������̼�ᷴӦ���������������������ӷ�Ӧ����ʽΪ��AlO2-+CO2+2H2O=Al��OH��3+HCO3-���ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3+HCO3-��
��7�����������������Ӧ�������ᱵ�����ӷ���ʽ��Ba2++SO42-=BaSO4�����ʴ�Ϊ��Ba2++SO42-=BaSO4����
���������⿼�����ӵļ��飬���ö���ʵ��Ͷ�������������ϵ�ģʽ�������˽����Ѷȣ�ͬʱ�漰���ӹ��桢���ӷ�Ӧ�ȶ��ǽ�����ע�����Ϣ��������K+��ȷ���׳���ʧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
���л�ѧ��Ӧ�У������ڵ�������ԭ��Ӧ�ǣ�������
| A��CaO+H2O�TCa��OH��2 | ||||
| B��2Na2O2+2H2O�T4NaOH+O2�� | ||||
| C��2Na+2H2O�T2NaOH+H2�� | ||||
D��C+H2O
|
���ںϳɰ���ҵ������˵����ȷ���ǣ�������
| A���Ӻϳ������������壬���а���һ��ռ15%�������������Ĺ�ҵ��Ч�ʶ��ܵ� |
| B������NH3��Һ����N2��H2��ѭ��ʹ�ã����ܵ�˵�����IJ��ʺܸ� |
| C���ϳɰ���ҵ�ķ�Ӧ�¶ȿ�����400��500�����ң�����Ϊ�������°��IJ������ |
| D���ϳɰ���ҵ����10 MPa��30MPa������������´����Ļ������ |
��֪X��Y������Ԫ�أ�IΪ�����ܣ���λ��kJ/mol��������±����������жϣ�������ǣ�������
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4 600 | 6 900 | 9 500 |
| Y | 580 | 1 800 | 2 700 | 11 600 |
| A��X�ĵ��ʳ���������ˮ���ҷ�Ӧ |
| B����Ԫ��Y���ڵ�3���ڣ�������NaOH��Һ��Ӧ |
| C��Ԫ��X�����γɻ�����ʱ����ѧʽΪXCl |
| D����Ԫ��Y���ڵ�3���ڣ��������γɵĻ�����Ϊ���ӻ����� |
��������������ͨ�����и���Һ�У�������������������ԭ��Ӧ�����ܴ���������ǣ�������
| A��Ba2+��Na+��NH4+��Br-��Cl- |
| B��K+��Na+��AlO2-��SO42 -��SiO32 - |
| C��Na+��HCO3-��SO32 -��CH3COO- |
| D��H+��Fe3+��Na+��NO3-��SO32 - |
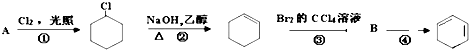



 ��R��R��Ϊ�������⣩
��R��R��Ϊ�������⣩ +R2OH
+R2OH +HCl��R��R��������
+HCl��R��R��������