��Ŀ����
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
| ������ | K����Ag����Mg2����Cu2����Al3����NH4+ |
| ������ | Cl����CO32����NO3����SO42����I�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������___________________________________��
(2)���м�������������ɫ��������ӷ���ʽ��________________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�________�ԣ�ԭ����____________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ____________________________����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
(1)K����NH4+��Cu2��
(2)6I����2NO3����8H��=3I2��2NO����4H2O
(3)Mg2����Al3����Cl����NO3����SO42����I�����ᡡMg2����2H2O??Mg(OH)2��2H����Al3����3H2O Al(OH)3��3H��
Al(OH)3��3H��
(4)Mg2����2OH��=Mg(OH) 2����Al3����4OH��=AlO2����2H2O��0.4
����

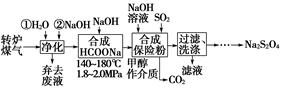
�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�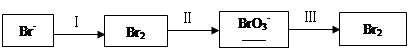
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol��L-1������,�����Һ�е�C ��HC
��HC ��Al
��Al ��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
A��ԭ�����Һ�е�C ��Al ��Al �����ʵ���֮��Ϊ1��2 �����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��5 |
| C��M��ʱ���ɵ�CO2Ϊ0.05 mol |
D��a�߱�ʾ�����ӷ���ʽΪ:Al +H++H2O +H++H2O Al(OH)3�� Al(OH)3�� |
 ��
�� ������϶��ɡ�Ϊ��ȷ�������ֻ�����ijɷ֣�ijͬѧ����������ʵ�������
������϶��ɡ�Ϊ��ȷ�������ֻ�����ijɷ֣�ijͬѧ����������ʵ�������