��Ŀ����
����˵�����ʾ������ȷ�ģ� ��
A����֪C(s)��O2(g)===CO2(g)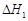 ��C(s)�� ��C(s)��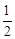 O2(g)===CO (g) O2(g)===CO (g)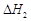 ���� ����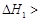 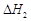 |
B����ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l) 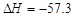 kJ��mol-1��������0.5molH2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ kJ��mol-1��������0.5molH2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ |
C����C(ʯī)===C(���ʯ)��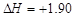 kJ��mol-1��֪�����ʯ��ʯī�ȶ� kJ��mol-1��֪�����ʯ��ʯī�ȶ� |
| D����֪�����ı�ȼ����Ϊ-285.8 kJ��mol-1����Ӧ���Ȼ�ѧ����ʽΪ2H2(g) + O2(g)��2H2O(l) ��H = �C285.8kJ��mol-1 |
B
̼��ȫȼ�ձȲ���ȫȼ�շų��������࣬���H1��>���H2��A�����к���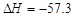 kJ��mol-1������Ũ��������ˮҪ���ȣ�B��ȷ����C(ʯī)=== C(���ʯ)��
kJ��mol-1������Ũ��������ˮҪ���ȣ�B��ȷ����C(ʯī)=== C(���ʯ)��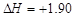 kJ��mol-1��֪ʯī�������Ƚ��ʯ�ͣ����ȶ���C���������ı�ȼ����Ϊ-285.8 kJ��mol-1ָ����1mol����ȼ�շų����ȣ�D����
kJ��mol-1��֪ʯī�������Ƚ��ʯ�ͣ����ȶ���C���������ı�ȼ����Ϊ-285.8 kJ��mol-1ָ����1mol����ȼ�շų����ȣ�D����
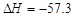 kJ��mol-1������Ũ��������ˮҪ���ȣ�B��ȷ����C(ʯī)=== C(���ʯ)��
kJ��mol-1������Ũ��������ˮҪ���ȣ�B��ȷ����C(ʯī)=== C(���ʯ)��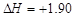 kJ��mol-1��֪ʯī�������Ƚ��ʯ�ͣ����ȶ���C���������ı�ȼ����Ϊ-285.8 kJ��mol-1ָ����1mol����ȼ�շų����ȣ�D����
kJ��mol-1��֪ʯī�������Ƚ��ʯ�ͣ����ȶ���C���������ı�ȼ����Ϊ-285.8 kJ��mol-1ָ����1mol����ȼ�շų����ȣ�D����
��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ
 �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪�� ====
==== ��H=��64kJ/mol
��H=��64kJ/mol ====
==== ��H= -196kJ/mol
��H= -196kJ/mol ="==="
="==="  ��H= -286kJ/mol
��H= -286kJ/mol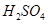 ��Һ��
��Һ�� ��
�� ��Ӧ����
��Ӧ���� ��
�� ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ �� ��3.0 mol/L��
��3.0 mol/L�� ��
�� ��Һ�м���һ������
��Һ�м���һ������ ��
�� ��Һ�����ȣ�����
��Һ�����ȣ����� �������Ʊ�
�������Ʊ�