��Ŀ����
��7�֣���1���� �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪�� ====
==== ��H=��64kJ/mol
��H=��64kJ/mol
 ====
==== ��H= -196kJ/mol
��H= -196kJ/mol
 ="==="
="==="  ��H= -286kJ/mol
��H= -286kJ/mol
��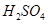 ��Һ��
��Һ�� ��
�� ��Ӧ����
��Ӧ���� ��
�� ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ ��
��2����������������ͬ��ӡˢ��·��Ľ�����ĩ��10�G ��3.0 mol/L��
��3.0 mol/L��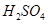 �����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
�����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
��3�����ᴿ��� ��Һ�м���һ������
��Һ�м���һ������ ��
�� ��Һ�����ȣ�����
��Һ�����ȣ����� �������Ʊ�
�������Ʊ� �����ӷ���ʽ�� ��
�����ӷ���ʽ�� ��
 �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪�� ====
==== ��H=��64kJ/mol
��H=��64kJ/mol ====
==== ��H= -196kJ/mol
��H= -196kJ/mol ="==="
="==="  ��H= -286kJ/mol
��H= -286kJ/mol��
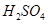 ��Һ��
��Һ�� ��
�� ��Ӧ����
��Ӧ���� ��
�� ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ ����2����������������ͬ��ӡˢ��·��Ľ�����ĩ��10�G
 ��3.0 mol/L��
��3.0 mol/L��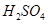 �����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
�����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����| | �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| | ͭƽ���ܽ����� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
| | �� �� �� | | | | | | | |
��3�����ᴿ���
 ��Һ�м���һ������
��Һ�м���һ������ ��
�� ��Һ�����ȣ�����
��Һ�����ȣ����� �������Ʊ�
�������Ʊ� �����ӷ���ʽ�� ��
�����ӷ���ʽ�� ��(1) Cu(s)+H2O2 (l)+2H��(aq)=Cu2��(aq)+2H2O(l) ��H=-320.KJ.mol-1��3�֣�
(2)H2O2�ֽ����ʼӿ� ��2�֣�
(3)2Cu2��+SO32-+2Cl��+H2O 2CuCl��+SO42-+2H����2�֣�
2CuCl��+SO42-+2H����2�֣�
(2)H2O2�ֽ����ʼӿ� ��2�֣�
(3)2Cu2��+SO32-+2Cl��+H2O
 2CuCl��+SO42-+2H����2�֣�
2CuCl��+SO42-+2H����2�֣���1�������˹���ɵ�Ӧ�á���
�� ====
==== ��H=��64kJ/mol��
��H=��64kJ/mol��
�� ====
==== ��H= -196kJ/mol��
��H= -196kJ/mol��
�� ="==="
="==="  ��H= -286kJ/mol֪���١�2���ڣ��ۡ�
��H= -286kJ/mol֪���١�2���ڣ��ۡ�
2�ɵ�Cu(s)+H2O2 (l)+2H��(aq)=Cu2��(aq)+2H2O(l) ��H=-320.KJ.mol��1����2��H2O2���ȶ��������ֽ⣬��������¶ȵ����ߣ�H2O2�ķֽ����ʻ����������Ũ�Ƚ��ͣ���ͭ��ƽ���ܽ����ʻ����ŷ�Ӧ�¶����߶��½���
��3��CuSO4����CuCl˵��Cu�Ļ��ϼ۽����ˣ���CuSO4�����������õ�1�����ӣ�Na2SO3����ԭ��ʧȥ2�����ӣ�������������Na2SO4�����Է�Ӧ�ķ���ʽΪ��2Cu2��+SO32-+2Cl��+H2O 2CuCl��+SO42-+2H����
2CuCl��+SO42-+2H����
��
 ====
==== ��H=��64kJ/mol��
��H=��64kJ/mol����
 ====
==== ��H= -196kJ/mol��
��H= -196kJ/mol����
 ="==="
="==="  ��H= -286kJ/mol֪���١�2���ڣ��ۡ�
��H= -286kJ/mol֪���١�2���ڣ��ۡ�
|
��3��CuSO4����CuCl˵��Cu�Ļ��ϼ۽����ˣ���CuSO4�����������õ�1�����ӣ�Na2SO3����ԭ��ʧȥ2�����ӣ�������������Na2SO4�����Է�Ӧ�ķ���ʽΪ��2Cu2��+SO32-+2Cl��+H2O
 2CuCl��+SO42-+2H����
2CuCl��+SO42-+2H����
��ϰ��ϵ�д�
�����Ŀ
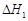 ��C(s)��
��C(s)��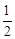 O2(g)===CO (g)
O2(g)===CO (g)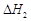 ����
����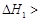
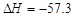 kJ��mol-1��������0.5molH2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ
kJ��mol-1��������0.5molH2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ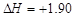 kJ��mol-1��֪�����ʯ��ʯī�ȶ�
kJ��mol-1��֪�����ʯ��ʯī�ȶ�