��Ŀ����
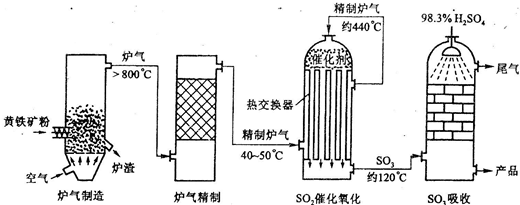
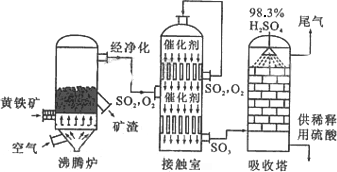
�Ի�����Ϊԭ����������Ĺ�������ͼ���£�

��1����ȼ�ջ�����Ļ�ѧ����ʽ��������
4______+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2
��2���Ӵ����з�����Ӧ�Ļ�ѧ����ʽ��______��
��3�����ݹ�������ͼ�ж�����˵����ȷ���ǣ�ѡ�������ĸ��______��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
��4��ÿ160g SO3������H2O���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ����ʽ��______��
��5���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Σ�
��SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ��______��
��Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ���뵽50.00mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ�������
���ֲⶨ�����
�������Ϊ10.00g��20.00gʱ��Ũ�������ӵ�������ͬ��
�������Ϊ30.00gʱ��Ũ�������ӵ�����Ϊ0.68g���������Ϊ40.00gʱ��Ũ������������䣮
���㣺������е�Ԫ�ص�����������______%�����������Ϊl5.00g��Ũ�������ӵ�����Ϊ______����������������λС����
�⣺��1���������⣬��������������ĵ�һ�������ԭ�ϣ��ɳɷֿ�֪��ѧʽΪFeS2���ʴ�Ϊ��FeS2��
��2������¯�����ɵĶ�����������Ϳ����е������ڽӴ����ڷ����Ĵ�������Ӧ������������2SO2+O2 2SO3 �ʴ�Ϊ��2SO2+O2
2SO3 �ʴ�Ϊ��2SO2+O2 2SO3��
2SO3��
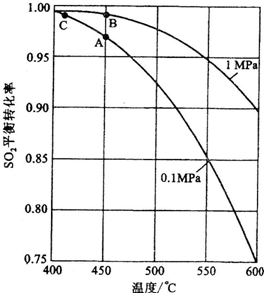
��3�������ʯ����Ӵ������߷�Ӧ���ʣ����ӿ���������ʹƽ��������У�����˶��������ת���ʣ�����ֻ�ı����ʲ��ı�ƽ�⣬���ı�ת���ʣ����������еĿ����к���������������������ȷ����abd���ʴ�Ϊ��abd��
��4��ÿ160g SO3�������ʵ���Ϊ2mol����Һ̬H2O���Ϸų�260.6kJ��������1mol���������ˮ��Ӧ����130.3KJ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
��5����SO2����Br2�ķ�Ӧ�ж�����������Ϊ���ᣬ�嵥�ʱ���ԭΪ�廯�⣬���ӷ���ʽΪSO2+Br2+2H2O=4H++2Br-+SO42-���ʴ�Ϊ��SO2+Br2+2H2O=4H++2Br-+SO42-��
�ڴ�������������������һ������ˮ���գ��õ�����β�Ʒ�ǣ�NH4��2SO3��NH4HSO3�Ļ�������Ӧ�����ǣ�OH-�����Ǻ�NH4HSO3�е�H+��Ӧ������ж��OH-�ٺ�NH4+��Ӧ�ų�����������������ε���������NH4HSO3����Ҳ���ų��İ���������Ϊ0��
����֪�������Ϊ30.00gʱ������0.04molNH3���������NH4HSO4����NaOH��Һ��Ӧ��2NH4HSO4+2NaOH=��NH4��2SO4+Na2SO4+H2O��ֻ�е�NH4HSO4�е�H+������ȫ��NH4+������NaOH��Һ��Ӧ����NH3��NH4++OH-=NH3��+H2O���ݴ��ж��������Ϊ10.00gʱNaOH��Һ�������������Ϊ20.00g��30.00gʱ�����ĵ�NaOH������ȣ���10.00g�����NH4HSO4 �루NH4��2SO4�����ʵ����ֱ�ΪX��Y��n��NH3��=n��OH-��-n��H+�������У�
���3X+2Y=3X+0.04�����Y=0.02mol����115X+132Y=10.00����X=0.064mol���������е�Ԫ�ص���������= ��100%=14.56%������15.00 g�����NaOH��Һ��Ӧ������NH3�����������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g��
��100%=14.56%������15.00 g�����NaOH��Һ��Ӧ������NH3�����������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g��
�ʴ�Ϊ��14.56�� 2.31g��
��������1�����ù�ҵ�������ԭ����������Ҫ��ԭ���ǻ�����
��2���Ӵ����ڵķ�Ӧ�Ƕ�������Ĵ�������
��3������Ӱ�췴Ӧ���ʵ����غ�ƽ���ƶ���Ӱ�����ط������
��4��ÿ160g SO3�������ʵ���Ϊ2mol����Һ̬H2O���Ϸų�260.6kJ�������������Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�Ͷ�Ӧ���ķ�Ӧ�ȣ�
��5���������������嵥�����õ��Ƕ�������Ļ�ԭ�Ժ��嵥�ʵ������ԣ�����������ԭ��Ӧ��д���ӷ���ʽ��
��6������Ӧ�����ǣ�OH-�����Ǻ�NH4HSO3�е�H+��Ӧ������ж��OH-�ٺ�NH4+��Ӧ�ų�����������������ε���������NH4HSO3����Ҳ���ų��İ���������Ϊ0��Ũ�������ӵ��������ǰ�������������һ�κ͵ڶ��ηų��İ���������һ���ģ�����˵��һ�ο϶���OH-�������������õڶ��ε������㣨��Ϊ��OH-���㣩��
���������⿼���˹�ҵ������Ļ���ԭ�������黯ѧƽ���Ӱ�����غͻ�ѧ��Ӧ���ʵ�Ӱ�����أ������Ṥҵβ��������Ϊ���忼�黯ѧ���㣬�漰�������㣬��������ļ��㡢��Χ�����ͼ��㡢��ϢǨ���ͼ��㡢NH4+��H+��NaOH��Һ��Ӧ���Ⱥ�˳���֪ʶ�������붨�����ϣ��ۺ���ǿ���ѶȽϴ�
��2������¯�����ɵĶ�����������Ϳ����е������ڽӴ����ڷ����Ĵ�������Ӧ������������2SO2+O2
 2SO3 �ʴ�Ϊ��2SO2+O2
2SO3 �ʴ�Ϊ��2SO2+O2 2SO3��
2SO3����3�������ʯ����Ӵ������߷�Ӧ���ʣ����ӿ���������ʹƽ��������У�����˶��������ת���ʣ�����ֻ�ı����ʲ��ı�ƽ�⣬���ı�ת���ʣ����������еĿ����к���������������������ȷ����abd���ʴ�Ϊ��abd��
��4��ÿ160g SO3�������ʵ���Ϊ2mol����Һ̬H2O���Ϸų�260.6kJ��������1mol���������ˮ��Ӧ����130.3KJ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
��5����SO2����Br2�ķ�Ӧ�ж�����������Ϊ���ᣬ�嵥�ʱ���ԭΪ�廯�⣬���ӷ���ʽΪSO2+Br2+2H2O=4H++2Br-+SO42-���ʴ�Ϊ��SO2+Br2+2H2O=4H++2Br-+SO42-��
�ڴ�������������������һ������ˮ���գ��õ�����β�Ʒ�ǣ�NH4��2SO3��NH4HSO3�Ļ�������Ӧ�����ǣ�OH-�����Ǻ�NH4HSO3�е�H+��Ӧ������ж��OH-�ٺ�NH4+��Ӧ�ų�����������������ε���������NH4HSO3����Ҳ���ų��İ���������Ϊ0��
����֪�������Ϊ30.00gʱ������0.04molNH3���������NH4HSO4����NaOH��Һ��Ӧ��2NH4HSO4+2NaOH=��NH4��2SO4+Na2SO4+H2O��ֻ�е�NH4HSO4�е�H+������ȫ��NH4+������NaOH��Һ��Ӧ����NH3��NH4++OH-=NH3��+H2O���ݴ��ж��������Ϊ10.00gʱNaOH��Һ�������������Ϊ20.00g��30.00gʱ�����ĵ�NaOH������ȣ���10.00g�����NH4HSO4 �루NH4��2SO4�����ʵ����ֱ�ΪX��Y��n��NH3��=n��OH-��-n��H+�������У�
| �������/g | 10.00 | 20.00 | 30.00 | 40.00 |
| ��NH4HSO4����NH4��2SO4/mol | X��Y | 2X��2Y | 3X��3Y | 4X��4Y |
| ����NH3/mol | X+2Y | X+2Y | 0.04 | 0 |
| ����NaOH/mol | 2X+2Y | 3X+2Y | 3X+0.04 | 3X+0.04 |
 ��100%=14.56%������15.00 g�����NaOH��Һ��Ӧ������NH3�����������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g��
��100%=14.56%������15.00 g�����NaOH��Һ��Ӧ������NH3�����������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g���ʴ�Ϊ��14.56�� 2.31g��
��������1�����ù�ҵ�������ԭ����������Ҫ��ԭ���ǻ�����
��2���Ӵ����ڵķ�Ӧ�Ƕ�������Ĵ�������
��3������Ӱ�췴Ӧ���ʵ����غ�ƽ���ƶ���Ӱ�����ط������
��4��ÿ160g SO3�������ʵ���Ϊ2mol����Һ̬H2O���Ϸų�260.6kJ�������������Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�Ͷ�Ӧ���ķ�Ӧ�ȣ�
��5���������������嵥�����õ��Ƕ�������Ļ�ԭ�Ժ��嵥�ʵ������ԣ�����������ԭ��Ӧ��д���ӷ���ʽ��
��6������Ӧ�����ǣ�OH-�����Ǻ�NH4HSO3�е�H+��Ӧ������ж��OH-�ٺ�NH4+��Ӧ�ų�����������������ε���������NH4HSO3����Ҳ���ų��İ���������Ϊ0��Ũ�������ӵ��������ǰ�������������һ�κ͵ڶ��ηų��İ���������һ���ģ�����˵��һ�ο϶���OH-�������������õڶ��ε������㣨��Ϊ��OH-���㣩��
���������⿼���˹�ҵ������Ļ���ԭ�������黯ѧƽ���Ӱ�����غͻ�ѧ��Ӧ���ʵ�Ӱ�����أ������Ṥҵβ��������Ϊ���忼�黯ѧ���㣬�漰�������㣬��������ļ��㡢��Χ�����ͼ��㡢��ϢǨ���ͼ��㡢NH4+��H+��NaOH��Һ��Ӧ���Ⱥ�˳���֪ʶ�������붨�����ϣ��ۺ���ǿ���ѶȽϴ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ