��Ŀ����
12��ij�о�С��̽����һ�ֺ����Һ����Na+��Cl-��I-��I2���Ʊ�NaI���壮����Ƶ�ʵ��������ͼ��
�����ϲ��ĵ�֪��2I-+2Cu2++SO32-+H2O�T2CuI��+SO42-+2H+
�ش��������⣺
��1���������ǹ��ˣ�
��2������ٷ�����Ӧ�����ӷ���ʽ�Ǽ����������ƣ�����I2+SO32-+H2O=2I-+SO42-+2H+����
��3����ʵ���в������Һ��ͨ����������ͨ��ϵ�в����ķ�����õ�Ƭ�����������ɵ�ⱥ��ʳ��ˮ�����Ʊ��������ӷ���ʽ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-��
��4������B��ֻ��������Ԫ�أ�����������m��Fe����m��I��=21��127���仯ѧʽ��Fe3I8��2FeI3•FeI2��
��5������ݷ����ķ�Ӧ�У������˺�ɫ��������������ɫ��������ʣ��÷�Ӧ�Ļ�ѧ����ʽ��8NaHCO3+Fe3I8=8NaI+Fe3O4+8CO2��+4H2O��
���� �ټ����������ƣ�����I2+SO32-+H2O=2I-+SO42-+2H+�������еķ�Ӧ�ڷ�����Ӧ��2I-+2Cu2++SO32-+H2O�T2CuI��+SO42-+2H+��������õ��⻯��ͭ�������⻯��ͭ���Ա���������Ϊ�ⵥ�ʣ��ⵥ���м�������ˮ����õ�������B����һ�ֺ�����Ԫ�غ͵�Ԫ�صĻ�����������м���̼��������Һ�����˿��Եõ��绰�ǵ���Һ���Ӷ��Ƶõ⻯�ƹ��壮�Դ˽����⣮
��� �⣺��1������Һ�з����CuI���壬Ӧ�ù��˵ķ������ʴ�Ϊ�����ˣ�
��2�������������ƣ������������Ʒ���������ԭ��Ӧ������I2+SO32-+H2O=2I-+SO42-+2H+���ʴ�Ϊ��I2+SO32-+H2O=2I-+SO42-+2H+��
��3����ⱥ��ʳ��ˮ�������������������������ƣ���Ӧ�����ӷ���ʽΪ2Cl-+2H2O $\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-���ʴ�Ϊ��2Cl-+2H2O $\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-��
��4��B��ֻ��������Ԫ�أ�����������m��Fe����m��I��=21��127����n��Fe����n��I��=$\frac{21}{56}$��$\frac{127}{127}$=3��8����ѧʽΪFe3I8 ��2FeI3•FeI2���ʴ�Ϊ��Fe3I8 ��2FeI3•FeI2��
��5����⻯������Һ�м���̼��������Һ�����ɺ�ɫ���������������Ͷ�����̼��ɫ���壬�����ķ�ӦΪ��8NaHCO3+Fe3I8=8NaI+Fe3O4+8CO2��+4H2O��
�ʴ�Ϊ��8NaHCO3+Fe3I8=8NaI+Fe3O4+8CO2��+4H2O��
���� �����ۺϿ������ʵ��Ʊ���Ϊ��Ƶ���㣬��Ŀ�漰���ʵķ�����ᴿ��ʵ�鷽������⣬���ؿ���ѧ�������ͽ�������������ע��������ʵ������Լ�ʵ�����̵ķ������Ѷ��еȣ�

| A�� | 100mL 1mol/LAlCl3��Һ | B�� | 200 mL 1mol/LMgCl2��Һ | ||
| C�� | 100mL 0.5mol/LCaCl2��Һ | D�� | 100 mL 2 mol/L KCl��Һ |
| A�� | ˮ�����[H+]�����¶ȵ����߶����� | B�� | ��35��ʱ��ˮ�����[H+]��[OH-] | ||
| C�� | ˮ�ĵ���̶ȣ�35�棾25�� | D�� | ˮ�����[OH-]���¶����߶����� |
| A�� | CH4 | B�� | Al | C�� | HCl | D�� | ϡH2SO4 |
| A�� | ��ˮ | B�� | ���� | C�� | �ƾ� | D�� | �� |
| A�� | ������Ľṹ��ʽ��C5H12 | B�� | ����ı���ģ�ͣ� | ||
| C�� | ���Ȼ�̼�ĵ���ʽ�� | D�� | ��ϩ�Ľṹʽ�� |
| A�� | ��ʱa+b��ֵ�Ǵ���14 | |
| B�� | ��Ӧ����Һ������ | |
| C�� | ��Ӧ����Һ����ˮ�����c��H+��С��10-7mol/L | |
| D�� | ��Ӧǰ�����NaOH��ˮ�ĵ���Ӱ��̶�һ�� |

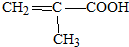 ��
��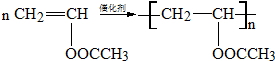 ��
��
 D���Է�������ת����
D���Է�������ת���� ϵ��
ϵ��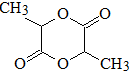 ����д��N������ȥ��Ӧ�Ļ�ѧ����ʽCH3CH��OH��COOH$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
����д��N������ȥ��Ӧ�Ļ�ѧ����ʽCH3CH��OH��COOH$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��