��Ŀ����
X��Z��Y��W��Ԫ�����ڱ�ǰ�����ڳ���������Ԫ�أ���ԭ��������������X�ĵ��ʼ��ЦҼ����Цм�����X�ĵ�һ�����ܱ�������Ԫ�ض���Y�ĵ縺�Ա�XС�����̬ԭ��������p�����Ǹò�s���ӵ�2����Z�Ƕ�������ԭ�Ӱ뾶��������Ԫ�أ�W��һ�ֺ��ص���������56����������30��
��1��Ԫ��X�����ڱ���λ�� ��W3+�ĺ�������Ų�ʽ�� ��
��2��Y��������YO2��H2O�Ƚϣ��۵�ϸߵ��� ���ѧʽ����Z���⻯������ ���壻Z2O2�Ļ�ѧ���������� ��
��3�����������£�Y�ĵ�����������X������������Ӧ��ˮ�����Ũ��Һ��Ӧ������ Y����ۺ����ᣬд���˷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����25�桢101kPa�£�W�ĵ�����������ȼ�պ�ָ���ԭ�¶Ⱥ�ѹǿ��ƽ��ÿת��1mol���ӷų�Q kJ����������W����ȼ�յ��Ȼ�ѧ����ʽ�� ��
��1��Ԫ��X�����ڱ���λ��
��2��Y��������YO2��H2O�Ƚϣ��۵�ϸߵ���
��3�����������£�Y�ĵ�����������X������������Ӧ��ˮ�����Ũ��Һ��Ӧ������ Y����ۺ����ᣬд���˷�Ӧ�Ļ�ѧ����ʽ��
��4����25�桢101kPa�£�W�ĵ�����������ȼ�պ�ָ���ԭ�¶Ⱥ�ѹǿ��ƽ��ÿת��1mol���ӷų�Q kJ����������W����ȼ�յ��Ȼ�ѧ����ʽ��
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������X��Y��Z��W��Ԫ�����ڱ�ǰ�����ڳ���������Ԫ�أ�ԭ��������������X�ĵ��ʼ��ЦҼ����Цм����䵥�ʺ���˫����������Ϊ�ǽ���Ԫ�أ���X�ĵ�һ�����ܱ�������Ԫ�ض�����Χ�����Ų�Ϊns2np3����XΪ��Ԫ�أ�Z�Ƕ�������ԭ�Ӱ뾶��������Ԫ�أ���ZΪNaԪ�أ�W��һ�ֺ��ص���������56����������30��������Ϊ56-30=26����WΪFeԪ�أ�Y��̬ԭ��������p�����Ǹò�s���ӵ���������Χ�����Ų�Ϊns2np4��Y�ĵ縺�Ա�X��С��Y��������X����ͬһ���ڣ�ֻ�ܴ��ڵ������ڣ���YΪSԪ�أ��ݴ˽��
���
�⣺X��Y��Z��W��Ԫ�����ڱ�ǰ�����ڳ���������Ԫ�أ�ԭ��������������X�ĵ��ʼ��ЦҼ����Цм����䵥�ʺ���˫����������Ϊ�ǽ���Ԫ�أ���X�ĵ�һ�����ܱ�������Ԫ�ض�����Χ�����Ų�Ϊns2np3����XΪ��Ԫ�أ�Z�Ƕ�������ԭ�Ӱ뾶��������Ԫ�أ���ZΪNaԪ�أ�W��һ�ֺ��ص���������56����������30��������Ϊ56-30=26����WΪFeԪ�أ�Y��̬ԭ��������p�����Ǹò�s���ӵ���������Χ�����Ų�Ϊns2np4��Y�ĵ縺�Ա�X��С��Y��������X����ͬһ���ڣ�ֻ�ܴ��ڵ������ڣ���YΪSԪ�أ�
��1��XΪNԪ�أ�ԭ����2�����Ӳ㣬����������Ϊ5��λ��Ԫ�����ڱ��ڶ����ڵ�VA�壬
W3+ΪFe3+��Feԭ��ʧȥ4s�ܼ���2�����ӡ�3d�ܼ�1�������γ�Fe3+��Fe3+��������Ų�ʽ��1s22s22p63s23p63d5��
�ʴ�Ϊ���ڶ����ڵ�VA�壻1s22s22p63s23p63d5��
��2��ͨ��������SO2�����壬H2O��Һ�壬�۵�ϸߵ���H2O��NaH�������Ӿ��壬Na2O2�������������������֮���γ����Ӽ�����������������ԭ��֮���γɹ��ۼ���
�ʴ�Ϊ��H2O�����ӣ����Ӽ������ۼ���
��3�����������£�S����������HNO3Ũ��Һ��Ӧ������H2SO4��HNO3����ԭΪNO2����Ӧ����ʽΪ��S+6HNO3��Ũ��
H2SO4+6NO2��+2H2O��
�ʴ�Ϊ��S+6HNO3��Ũ��
H2SO4+6NO2��+2H2O��
��4����25��C��1O1kPa�£�Fe�ĵ�����������ȼ������Fe3O4���ָ���ԭ�¶Ⱥ�ѹǿ��ƽ��ÿת��1mol���ӷų�QkJ����������3moFe��Ӧ�ų�������Ϊ
��QkJ=8QkJ��Fe����ȼ�յ��Ȼ�ѧ����ʽ��3Fe��s��+2O2��g��=Fe3O4��s����H=-8Q KJ/mol��
�ʴ�Ϊ��3Fe��s��+2O2��g��=Fe3O4��s����H=-8Q KJ/mol��
��1��XΪNԪ�أ�ԭ����2�����Ӳ㣬����������Ϊ5��λ��Ԫ�����ڱ��ڶ����ڵ�VA�壬
W3+ΪFe3+��Feԭ��ʧȥ4s�ܼ���2�����ӡ�3d�ܼ�1�������γ�Fe3+��Fe3+��������Ų�ʽ��1s22s22p63s23p63d5��
�ʴ�Ϊ���ڶ����ڵ�VA�壻1s22s22p63s23p63d5��
��2��ͨ��������SO2�����壬H2O��Һ�壬�۵�ϸߵ���H2O��NaH�������Ӿ��壬Na2O2�������������������֮���γ����Ӽ�����������������ԭ��֮���γɹ��ۼ���
�ʴ�Ϊ��H2O�����ӣ����Ӽ������ۼ���
��3�����������£�S����������HNO3Ũ��Һ��Ӧ������H2SO4��HNO3����ԭΪNO2����Ӧ����ʽΪ��S+6HNO3��Ũ��
| ||
�ʴ�Ϊ��S+6HNO3��Ũ��
| ||
��4����25��C��1O1kPa�£�Fe�ĵ�����������ȼ������Fe3O4���ָ���ԭ�¶Ⱥ�ѹǿ��ƽ��ÿת��1mol���ӷų�QkJ����������3moFe��Ӧ�ų�������Ϊ
3mol��
| ||
| 1mol |
�ʴ�Ϊ��3Fe��s��+2O2��g��=Fe3O4��s����H=-8Q KJ/mol��
���������⿼��ṹλ�����ʹ�ϵ����������Ų����ɡ�������ԭ��Ӧ���Ȼ�ѧ����ʽ��д�ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���ע�����X���ʻ�ѧ����Ԫ�ص�����ȷ��XΪ��Ԫ�أ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���������У���֤��ij������������ʵ��ǣ�������
| A���ۻ�ʱ���ܵ��� |
| B���������ӻ������ǹ��ۻ����� |
| C��ˮ��Һ�ĵ��������ܲ� |
| D����Һ�д��ڵ���ƽ�⣬����������Ӻ�δ����ķ��ӹ��� |
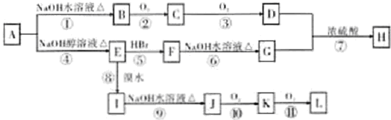
 ��R������
��RΪ������ �������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·����ͼ
�������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·����ͼ
 �۰��� ��18O ��
�۰��� ��18O �� ���� ��16O ������ ��H2O ��D2O
���� ��16O ������ ��H2O ��D2O ��д���ƾ���ͬ���칹������ѵĽṹʽ
��д���ƾ���ͬ���칹������ѵĽṹʽ