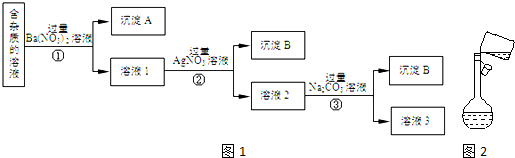
��Ŀ����
19���������ӷ���ʽ��ȷ���ǣ�������| A�� | Na��ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | 0.01mol/L NH4Al��SO4��2��Һ��0.02mol/L Ba��OH��2��Һ�������� NH4++Al3++2SO42-+2Ba2++4 OH-�T2 Ba SO4��+Al��OH��3��+NH3•H2O | |
| C�� | ��������Һ�������Һ��ϣ�SiO32-+2H+�TH2SiO3�� | |
| D�� | Ũ�����м���������۲����ȣ�Fe+3NO3-+6H+ $\frac{\underline{\;\;��\;\;}}{\;}$Fe3++3NO2��+3H2O |
���� A����ɲ��غ㣻
B�����ʵ�����Ϊ1��2����Ӧ�������ᱵ������������һˮ�ϰ���
C����Ӧ���ɹ���ʹ����ƣ����������ӷ�Ӧ�б�����ѧʽ��
D��Fe������������������������������ˮ��
��� �⣺A��Na��ˮ��Ӧ�����ӷ�ӦΪ2Na+2H2O�T2Na++2OH-+H2������A����
B.0.01mol/L NH4Al��SO4��2��Һ��0.02mol/L Ba��OH��2��Һ�������ϵ����ӷ�ӦΪNH4++Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+Al��OH��3��+NH3•H2O����B��ȷ��
C����������Һ�������Һ��ϵ����ӷ�ӦΪSiO32-+2CH3COOH�TH2SiO3��+22CH3COO-����C����
D��Ũ�����м���������۲����ȵ����ӷ�ӦΪFe+2NO3-+4H+ $\frac{\underline{\;\;��\;\;}}{\;}$Fe2++2NO2��+2H2O����D����
��ѡB��
���� ���⿼�����ӷ�Ӧ����ʽ��д�������жϣ�Ϊ��Ƶ���㣬����ϰ�����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ�������������ԭ��Ӧ�����ֽⷴӦ�����ӷ�Ӧ���飬ע�����ӷ�Ӧ�б�����ѧʽ�����ʼ����ӡ�����غ㣬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д�
�����Ŀ
9��SO2�ĺ����ǿ��������ձ���һ����Ҫ���ָ�꣬������ѧ֪ʶ�ش��������⣮
��1����ҵ����������У�SO2��������ԭ��Ϊ��
2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g��
T��ʱ����ij�ܱ������г���һ��SO2��g����O2��g��������������Ӧ�����SO2��g����ƽ��ת���ʣ�a������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ1��ʾ��

��a��b�����Ӧ��ƽ�ⳣ��K��a��=K��b�������������������=������ͬ����SO2Ũ��c��a����c��b����
��c��ʱ����Ӧ����v��������v���棩��
��2���绯ѧ������SO2��
���Ṥҵβ���е�SO2������������Ʊ����ᣬͬʱ��õ��ܣ�װ����ͼ2��ʾ���缫��Ϊ���Բ��ϣ���
��M�������ĵ缫��ӦʽΪSO2-2e-+2H2O=4H++SO42-��
����ʹ��װ�õĵ���ǿ�ȴﵽ2.0A��������ÿ����Ӧ��ͨ���״������������Ϊ0.014L����֪��1��e-��������Ϊ1.6��10-19C����
��3����Һ������SO2��
��֪������H2SO3��H2CO3�ĵ��볣�������ʾ��
�����£���SO2����ͨ��100mL 0.2mol•L-1��Na2CO3��Һ�У���ͨ��448mLSO2ʱ��������Ϊ��״���µ��������ͬ�������������ӷ���ʽΪSO2+H2O+CO32-=HCO3-+HSO3-����ͨ��896mLSO2ʱ��������Һ�������ԣ���Һ�и�����Ũ���ɴ�С��˳��Ϊc��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��1����ҵ����������У�SO2��������ԭ��Ϊ��
2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g��
T��ʱ����ij�ܱ������г���һ��SO2��g����O2��g��������������Ӧ�����SO2��g����ƽ��ת���ʣ�a������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ1��ʾ��

��a��b�����Ӧ��ƽ�ⳣ��K��a��=K��b�������������������=������ͬ����SO2Ũ��c��a����c��b����
��c��ʱ����Ӧ����v��������v���棩��
��2���绯ѧ������SO2��
���Ṥҵβ���е�SO2������������Ʊ����ᣬͬʱ��õ��ܣ�װ����ͼ2��ʾ���缫��Ϊ���Բ��ϣ���
��M�������ĵ缫��ӦʽΪSO2-2e-+2H2O=4H++SO42-��
����ʹ��װ�õĵ���ǿ�ȴﵽ2.0A��������ÿ����Ӧ��ͨ���״������������Ϊ0.014L����֪��1��e-��������Ϊ1.6��10-19C����
��3����Һ������SO2��
��֪������H2SO3��H2CO3�ĵ��볣�������ʾ��
| ���볣�� �� | K1 | K2 |
| H2SO3 | 1.3��10-2 | 6.3��10-8 |
| H2CO3 | 4.2��10-7 | 5.6��10-11 |
10�����й����л��������˵����ȷ���ǣ�������
| A�� | ���ڳ����¿�����ˮ����ȡ����Ӧ | |
| B�� | �������ܷ���������Ӧ��������Ӧ | |
| C�� | �Ҵ������ᶼ�����������Ʒ�Ӧ | |
| D�� | ���ࡢ��֬�͵����ʶ��ܷ���ˮ�ⷴӦ |
9�����ж�ijЩ���ӵļ���ʵ�飬������һ����ȷ���ǣ�������
| ѡ�� | ʵ�鷽�� | ���� | ���� |
| ��ij��Һ����AgNO3��Һ | ���ְ�ɫ���� | ԭ��Һ��һ����Cl- | |
| ��ij��Һ����BaCl2��Һ | ���ְ�ɫ���� | ԭ��Һ��һ����SO42- | |
| ��ij��Һ����NaOH��Һ | ������ɫ���� | ԭ��Һ��һ����Cu2+ | |
| ��ij��Һ����H2SO4��Һ | ������ɫ��ζ���� | ԭ��Һ��һ����CO32- |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ij��ȤС��ͬѧ�о�������Ԫ�ؼ���ijЩ������IJ������ʣ������������£�
ij��ȤС��ͬѧ�о�������Ԫ�ؼ���ijЩ������IJ������ʣ������������£�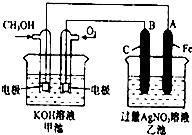 ��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O ��д���пհף�
��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O ��д���пհף� ��Na2O2��SO2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2SO2�T2Na2SO4��
��Na2O2��SO2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2SO2�T2Na2SO4��