��Ŀ����
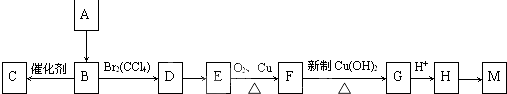
 �й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����������A��C��D�dz������壬A�ǵ��¡�����ЧӦ������Ҫ���ʣ�B�dz�����ɫҺ�壬C��ʹʪ��ĺ�ɫʯ����ֽ������F�ǰ�ɫ������G�Ǻ��ɫ������X��������A��B��C�����ʵ���֮��Ϊ1��1��1��
�й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����������A��C��D�dz������壬A�ǵ��¡�����ЧӦ������Ҫ���ʣ�B�dz�����ɫҺ�壬C��ʹʪ��ĺ�ɫʯ����ֽ������F�ǰ�ɫ������G�Ǻ��ɫ������X��������A��B��C�����ʵ���֮��Ϊ1��1��1����ش��������⣺
��1��C�ĵ���ʽΪ
��2��X�Ļ�ѧʽΪ
��3��д����Ӧ�ٵ����ӷ���ʽ��
��4��д����Ӧ�ڵĻ�ѧ����ʽ��
���㣺������ƶ�
ר�⣺�ƶ���
������������A��C��D�dz������壬A�ǵ��¡�����ЧӦ������Ҫ���ʣ���AΪCO2��C��ʹʪ��ĺ�ɫʯ����ֽ��������CΪNH3��B�dz�����ɫҺ�壬��BΪH2O��DΪO2��X�������ᷴӦ������Ӧ��X��������A��B��C�����ʵ���֮��Ϊ1��1��1�����A��B��C����ɿ�֪��XΪNH4HCO3��G�Ǻ��ɫ��������GΪFe��OH��3��F�ǰ�ɫ��������FΪFe��OH��2��Fe��OH��2��O2������Fe��OH��3���ݴ˴��⣮
���
�⣺������A��C��D�dz������壬A�ǵ��¡�����ЧӦ������Ҫ���ʣ���AΪCO2��C��ʹʪ��ĺ�ɫʯ����ֽ��������CΪNH3��B�dz�����ɫҺ�壬��BΪH2O��DΪO2��X�������ᷴӦ������Ӧ��X��������A��B��C�����ʵ���֮��Ϊ1��1��1�����A��B��C����ɿ�֪��XΪNH4HCO3��G�Ǻ��ɫ��������GΪFe��OH��3��F�ǰ�ɫ��������FΪFe��OH��2��Fe��OH��2��O2������Fe��OH��3��
��1��CΪNH3��C�ĵ���ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��XΪNH4HCO3���ʴ�Ϊ��NH4HCO3��
��3����Ӧ�ٵ����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH��+O2�����ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH��+O2����
��4����Ӧ�ڵĻ�ѧ����ʽΪ4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��1��CΪNH3��C�ĵ���ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��XΪNH4HCO3���ʴ�Ϊ��NH4HCO3��
��3����Ӧ�ٵ����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH��+O2�����ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH��+O2����
��4����Ӧ�ڵĻ�ѧ����ʽΪ4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
���������⿼�������ƶϣ���������A��C��DΪ��ɫ���壬C��ʹʪ��ĺ�ɫʯ����ֽ������A��������Ʒ�Ӧ����D���Լ�F��G����ɫ�����ƶ�ͻ�ƿڣ���Ҫѧ����������Ԫ�ػ��������ʣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
��ͼ��ʾ��ʵ���������ܴﵽʵ��Ŀ���ǣ�������
A�� ��Cl2������ |
B�� ��ȥSO2�е�HCl |
C�� ��ˮ���ռ�H2 |
D�� ʵ������ȡNH3 |
����ʽΪC5H12O�ı���һԪ���������Է���������ͬ�ı���һԪ�������������Ӧ�����ɵ������У������������칹����������
| A��13�� | B��14�� |
| C��15�� | D��16�� |


 ͼ�и����ʾ��ɳ���Ԫ�أ�ԭ��������18����ɣ���֪A��B��KΪ���ʣ����ڳ�����A��KΪ���壬BΪ���壮DΪ��������ɫҺ�壮I��һ�ֳ��õĻ��ʣ�����ˮ��Һ�еμ�AgNO3��Һ�в�����ϡHNO3�İ�ɫ����������J��һ�ֳ������õ�ζ�������ǵ��ת����ϵ��ͼ��ʾ��ͼ�з�Ӧ����δ�г�������ش��������⣺
ͼ�и����ʾ��ɳ���Ԫ�أ�ԭ��������18����ɣ���֪A��B��KΪ���ʣ����ڳ�����A��KΪ���壬BΪ���壮DΪ��������ɫҺ�壮I��һ�ֳ��õĻ��ʣ�����ˮ��Һ�еμ�AgNO3��Һ�в�����ϡHNO3�İ�ɫ����������J��һ�ֳ������õ�ζ�������ǵ��ת����ϵ��ͼ��ʾ��ͼ�з�Ӧ����δ�г�������ش��������⣺ ���и��Ϲ����ŵĸ����л�������ž��и��ԵĶ����ԣ��ڲ�ͬ�������������Ļ�ѧ���ʿɷֱ�Ӹ����������ۣ��磺�������������ŷֱ��ǣ������ƣ���
���и��Ϲ����ŵĸ����л�������ž��и��ԵĶ����ԣ��ڲ�ͬ�������������Ļ�ѧ���ʿɷֱ�Ӹ����������ۣ��磺�������������ŷֱ��ǣ������ƣ���