��Ŀ����
̽���⣺Ϊ�˲ⶨij�л���A�Ľṹ����������ʵ�飺
��1����2.3g���л�����ȫȼ�գ�����0.1molCO2��2.7gH2O��
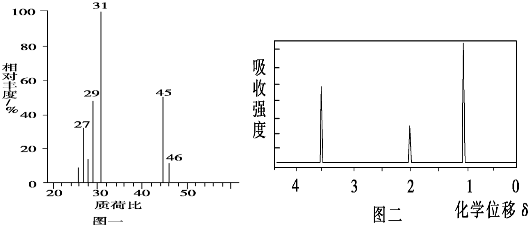
��2���������Dzⶨ����Է�������������ͼһ��ʾ������ͼ��
��3���ú˴Ź����Dzⶨ���л���õ���ͼ����ʾ��ͼ�ף�ͼ�����������֮����1��2��3���Իش��������⣺
��1���л���A����Է��������� ��
��2���л���A�ķ���ʽ ��
��3��д���л���A���ܵĽṹ��ʽ�� ��
��1����2.3g���л�����ȫȼ�գ�����0.1molCO2��2.7gH2O��
��2���������Dzⶨ����Է�������������ͼһ��ʾ������ͼ��
��3���ú˴Ź����Dzⶨ���л���õ���ͼ����ʾ��ͼ�ף�ͼ�����������֮����1��2��3���Իش��������⣺

��1���л���A����Է���������
��2���л���A�ķ���ʽ
��3��д���л���A���ܵĽṹ��ʽ��
���㣺�й��л������ʽȷ���ļ���
ר�⣺�л���������ͨʽ��Ӧ�ù���
��������1�������ʺϱȿ�֪�����л���A����Է�����Ϊ46��
��2������2.3g���л�����ȼ�����ɵĶ�����̼��ˮ�����ж��л���A�е�̼Ԫ�ء���Ԫ�ص����ʵ��������������ж��Ƿ�����Ԫ�أ������C��H��OԪ�ص����ʵ���֮�ȣ����ȷ��A�ķ���ʽ��
��3����Ϻ˴Ź��������жϸ��л�����ӵĽṹ��ʽ��
��2������2.3g���л�����ȼ�����ɵĶ�����̼��ˮ�����ж��л���A�е�̼Ԫ�ء���Ԫ�ص����ʵ��������������ж��Ƿ�����Ԫ�أ������C��H��OԪ�ص����ʵ���֮�ȣ����ȷ��A�ķ���ʽ��
��3����Ϻ˴Ź��������жϸ��л�����ӵĽṹ��ʽ��
���
�⣺��1����A������ͼ�У�����ʺɱ�Ϊ46����������Է�������Ҳ��46��
�ʴ�Ϊ��46��
��2��2.3 g���л����У�n��C��=n��CO2��=0.1 mol�����е�̼ԭ�ӵ�����Ϊm��C��=0.1 mol��12 g?mol-1=1.2 g��
��ԭ�ӵ����ʵ���Ϊ��n��H��=
��2=0.3 mol����ԭ�ӵ�����Ϊm��H��=0.3 mol��1 g?mol-1=0.3 g��
���л�����m��O��=2.3 g-1.2 g-0.3 g=0.8 g����Ԫ�ص����ʵ���Ϊn��O��=
=0.05 mol��
��n��C����n��H����n��O��=0.1 mol��0.3 mol��0.05 mol=2��6��1������A��ʵ��ʽ�ǣ�C2H6O��
��Ϊʵ��ʽ��C2H6O���л����У���ԭ�����Ѿ��ﵽ���ͣ�������ʵ��ʽ��Ϊ����ʽ��
�ʴ�Ϊ��C2H6O��
��3��A���������ֿ��ܵĽṹ��CH3OCH3��CH3CH2OH����Ϊǰ�ߣ����ں˴Ź���������Ӧֻ��1���壻��Ϊ���ߣ����ں˴Ź���������Ӧ��3���壬����3��������֮����1��2��3����ȻCH3CH2OH�������⣬����AΪ�Ҵ���
�ʴ�Ϊ��CH3CH2OH��
�ʴ�Ϊ��46��
��2��2.3 g���л����У�n��C��=n��CO2��=0.1 mol�����е�̼ԭ�ӵ�����Ϊm��C��=0.1 mol��12 g?mol-1=1.2 g��
��ԭ�ӵ����ʵ���Ϊ��n��H��=
| 2.7g |
| 18g/mol |
���л�����m��O��=2.3 g-1.2 g-0.3 g=0.8 g����Ԫ�ص����ʵ���Ϊn��O��=
| 0.8g |
| 16g/mol |
��n��C����n��H����n��O��=0.1 mol��0.3 mol��0.05 mol=2��6��1������A��ʵ��ʽ�ǣ�C2H6O��
��Ϊʵ��ʽ��C2H6O���л����У���ԭ�����Ѿ��ﵽ���ͣ�������ʵ��ʽ��Ϊ����ʽ��
�ʴ�Ϊ��C2H6O��
��3��A���������ֿ��ܵĽṹ��CH3OCH3��CH3CH2OH����Ϊǰ�ߣ����ں˴Ź���������Ӧֻ��1���壻��Ϊ���ߣ����ں˴Ź���������Ӧ��3���壬����3��������֮����1��2��3����ȻCH3CH2OH�������⣬����AΪ�Ҵ���
�ʴ�Ϊ��CH3CH2OH��
���������⿼�����л������ʽ���ṹ��ʽ�ļ��㷽������Ŀ�Ѷ��еȣ�ע����������غ�ȷ���л������ʽ�ķ�������ȷ�����ȡ��˴Ź������ĺ����ǽ���ؼ���

��ϰ��ϵ�д�
�����Ŀ
 ���������Ǻ��ǣ� ����ʯ��ʯī��
���������Ǻ��ǣ� ����ʯ��ʯī�� I�������ӵĿռ乹��Ϊ
I�������ӵĿռ乹��Ϊ
