��Ŀ����
6��ij��ѧ��ȤС������йص��ʳ��ˮ��̽��ʵ�飬���װ����ͼ1��ʾ��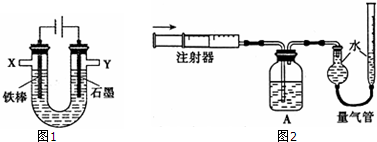
ʵ��һ����ⱥ��ʳ��ˮ��
��1���������Ʊ���ʳ��ˮ�IJ��������ձ��м���һ����������ˮ���߽������ʳ�ι��壬ֱ�����岻�ټ����ܽ�Ϊֹ
��2����ⱥ��ʳ��ˮ�����ӷ���ʽΪ2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2��
ʵ�������ⲻ����ʳ��ˮ�������������ͬ�����£����1mol•L-1NaCl��Һ���ռ��������������壮��X���ռ���V1mL���壬ͬʱ����Y���ռ���V2mL���壬ֹͣ��⣮�������V2��V1�������ⱥ��ʳ��ˮ��ȣ�
Y���ռ�����������ɫ���Խ�dz�������۷������������������ԭ���У�i���в���C12�ܽ���NaCl��Һ�У�ii����02���ɣ�
��3�����ʵ��֤���в���C12�ܽ���NaCl��Һ�У�ʵ�鷽��Ϊȡ����ʯī�缫������Һ�����ڵ���KI��ֽ�ϣ���ֽ������
��4��֤����O2���ɲ��ⶨO2�����������ͼ2��ʾװ�ý���ʵ�飮ͨ��ע���������ؽ���Y���ռ�����V2mL����ȫ������װ��A��ʢ�������Լ����У����գ����������ռ���V3mL���壨��V1��V2��V3������ͬ�����²�ã���
��װ��A����������ȫ����������
�ڱ�ʵ���У��۲쵽�����ܵ��Ҳ�Һ������������˵��ʯī�缫����02���ɣ�
��ʵ�����Ƿ���ҪԤ�ȳ���װ���еĿ���������ǡ�����
��5��ʵ����У���ʯī�缫������Cl2�������ΪV1-2V3mL���ô���ʽ��ʾ����
ʵ�鷴˼��
��6��������ʵ����֪����ͨ�����ʳ��ˮ�����ػ�ýϴ��������������ʱӦ���Ƶ����������ñ���ʳ��ˮ���ڿ���NaCl��Һ��Ũ����һ����Χ�ڣ�Ҫ��һ��֤�������ۣ�������е�ⲻͬŨ��ʳ��ˮ��ƽ��ʵ�飮
���� ��1������һ��ˮ�в������ܽ�ʳ�ι���ʱ���õ���Һ��Ϊ����ʳ��ˮ�����ƣ�
��2����ⱥ��ʳ��ˮ����Ϊ������ʧ������������������Ϊ�����ӵõ��������������ݴ���д��
��3������������ʹʪ��ĵ���KI��ֽ�����жϣ�
��4������YΪ�������ռ�����V2mL��������Ҫ������������֤����O2��������Ҫ��ȥ�������������ܵ��Ҳ�Һ����������˵�������������ɣ����ݱ����Ŀ�����ѹǿ��Ӱ�죬����ʵ���в���ҪԤ�ȳ���װ���еĿ�����
��5����������X���ռ���������ΪV1mL���壬����Y���ռ���Ϊ���������������ݣ�4���������������ռ���V3mL���弴����������ʯī�缫������Cl2�������Ϊxml������ݵ�����������ĵ�ʧ�����غ���㣻
��6��������ʵ����֪����ͨ�����ʳ��ˮ�����ػ�ýϴ�������������ʹ�ñ���ʳ��ˮ������С�������ܽ⣬�������л�Ҫ����NaCl��Һ��Ũ����һ����Χ�ڣ���֤�������������ݴ˷�����
��� �⣺��1��һ��ˮ�в������ܽ�ʳ�ι���ʱ���õ���Һ��Ϊ����ʳ��ˮ���������Ʊ���ʳ��ˮ�IJ���Ϊ���ձ��м���һ����������ˮ���߽������ʳ�ι��壬ֱ�����岻�ټ����ܽ�Ϊֹ���ʴ�Ϊ�����ձ��м���һ����������ˮ���߽������ʳ�ι��壬ֱ�����岻�ټ����ܽ�Ϊֹ��
��2����ⱥ��ʳ��ˮ����Ϊ������ʧ������������������Ϊ�����ӵõ����������������ӷ���ʽΪ2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2�����ʴ�Ϊ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2����
��3����Ϊ������ʹʪ��ĵ���KI��ֽ����������֤���в���C12�ܽ���NaCl��Һ�У���ȡ����ʯī�缫������Һ�����ڵ���KI��ֽ�ϣ���ֽ������˵������������
�ʴ�Ϊ��ȡ����ʯī�缫������Һ�����ڵ���KI��ֽ�ϣ���ֽ������
��4������ΪYΪ�������ռ�����V2mL��������Ҫ������������֤����O2��������Ҫ��ȥ��������װ��A����������ȫ�����������ʴ�Ϊ����ȫ����������
�ڵ������ܵ��Ҳ�Һ����������˵�������������ɣ��ʴ�Ϊ�������ܵ��Ҳ�Һ��������
�۱����Ŀ�����ѹǿ��Ӱ�죬����ʵ���в���ҪԤ�ȳ���װ���еĿ������ʴ�Ϊ����
��5����Ϊ����X���ռ���������ΪV1mL���壬����Y���ռ���Ϊ���������������ݣ�4���������������ռ���V3mL���弴����������ʯī�缫������Cl2�������Ϊxml������ݵ�����������ĵ�ʧ�����غ㣬V1��2=x��2+V3��4������x=V1-2V3���ʴ�Ϊ��V1-2V3��
��6��������ʵ����֪����ͨ�����ʳ��ˮ�����ػ�ýϴ�������������ʹ�ñ���ʳ��ˮ������С�������ܽ⣬�������л�Ҫ����NaCl��Һ��Ũ����һ����Χ�ڣ���֤�������������ʴ�Ϊ���ñ���ʳ��ˮ������NaCl��Һ��Ũ����һ����Χ�ڣ�
���� ������Ҫ�����˵�ⱥ��ʳ��ˮ�����̽�����漰����Һ�����ơ�����ʽ����д���缫����ļ����������ķ�����̽�����ۺ��Խ�ǿ���ѶȽϴ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�| A�� | CH3CH2CH2CH3 | B�� | CH3-CH=CHCH3 | C�� | HC��C-CH2 | D�� | CH3-C��C-C��C-CH3 |
| A�� | �����������䣬����ѹǿ��ƽ�ⲻ�����ƶ� | |
| B�� | �÷�Ӧʽ��nֵһ��Ϊ2 | |
| C�� | �����������䣬����ѹǿ�������������������� | |
| D�� | ԭ�������A��B�����ʵ���֮��Ϊ2��1����2M��A��+M��B��=3M��D��������M��ʾ���ʵ�Ħ�������� |
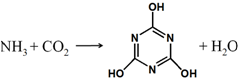 CO2����Դ�������ǽ������ЧӦ����Ҫ;������ͼ����һ����������NH3����CO2������Ҫ������Ʒ��������ķ�Ӧ�������й����������˵����ȷ���ǣ�������
CO2����Դ�������ǽ������ЧӦ����Ҫ;������ͼ����һ����������NH3����CO2������Ҫ������Ʒ��������ķ�Ӧ�������й����������˵����ȷ���ǣ�������| A�� | ����ʽΪC3H6N3O3 | B�� | �����мȺ��ЦҼ��ֺ��Цм� | ||
| C�� | �����мȺ����Լ����ֺ��Ǽ��Լ� | D�� | ���ɸ����ʵ�������ӦΪ�кͷ�Ӧ |
| A�� | ���һ�����Ǹ߷��ӻ������ˮ�������ͬ | |
| B�� | �ױ���ʹ���Ը��������Һ��ɫ��֤���ױ������д��ڵ�˫������Ľṹ | |
| C�� | ������ʳ��ƾ������ɵ��ۡ������ǡ��Ҵ��Ļ�ѧ�仯���� | |
| D�� | �״����Ҷ�����HOCH2CH2OH����Ϊͬϵ�� |
| A�� | ��NaAlO2��Һ��ͨ��������CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| B�� | ��NH4��2Fe��SO4��2��Һ�м�����������������Һ��NH4++SO42-+Ba2++OH-�TBaSO4��+H2O | |
| C�� | ��ҵ���ð�ˮ���ն�������2NH3��H2O+SO2�T2NH4++SO32- | |
| D�� | ��������������ϡ����3Fe2++4H++NO3-�T3Fe3++NO��+2H2 |


 ��
��
 ��28����ʵ��ʴ���ṩ��������ʾ�������д�������Ȼ��ˮ�������������1000�����Դ��Ҫ����Ȼ��ˮ�������־��壬������ƽ��ÿ46��ˮ���ӹ�����8������ÿ����������1��CH4���ӻ�1������H2O���ӣ�����������Ϣ������������⣺
��28����ʵ��ʴ���ṩ��������ʾ�������д�������Ȼ��ˮ�������������1000�����Դ��Ҫ����Ȼ��ˮ�������־��壬������ƽ��ÿ46��ˮ���ӹ�����8������ÿ����������1��CH4���ӻ�1������H2O���ӣ�����������Ϣ������������⣺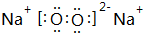 ��
�� ̼Ԫ���ǹ����л���Ļ���Ԫ�أ�
̼Ԫ���ǹ����л���Ļ���Ԫ�أ�