��Ŀ����
15�������ᣨH3PO3����Ԫ��ǿ�ᣩ�������ڹ�ũҵ������������Ҫ���ã���֪��25��ʱ�������ᣨH3PO3����Ka1=5��10-2��Ka2=2.5��10-7
��1�������ᣨH3PO3������ǿ��ԭ�ԣ��ɱ�����������Ϊ���ᣮ��÷�Ӧ�����ӷ���ʽΪH3PO3+2Ag++H2O=H3PO4+2Ag+2H+��
��2���Դӵ���ƽ���ƶ��ĽǶȽ���Ka1��Ka2����ԭ���������һ�����������������������������ĵڶ������룬���Ե�һ�������Ka1Զ���ڵڶ��������Ka2��
��3����ϡ��Һ��H3PO3��aq��?H2PO3-��aq��+H+��aq����H=akJ/mol
H2PO3-��aq��?HPO32-��aq��+H+��aq����H=bkJ/mol
H+��aq��+OH-��aq��=H2O��l����H=ckJ/mol
H3PO3��aq��+2NaOH��aq��?Na2HPO3��aq��+2H2O��l����H=a+b+2ckJ/mol
��4�����������ƿ�ʹ��ˮ��ɫ��25��ʱ��NaH2PO3ˮ�ⷴӦ��Kb=2��10-13������NaH2PO3��Һ�м���������I2������Һ��$\frac{c��{H}_{3}P{O}_{3}��}{c��{H}_{2}P{O}_{3}^{-}��}$������ �����������С�����䡱����
��5�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�

��aΪ��Դ�������������������Ʒ���з�Ӧ�����ӷ���ʽΪ2H++HPO32-=H3PO3��
�ڵõ�0.1mol�������ͬʱ��������B�ҿ��Ƶ�NaOH����Ϊ8g��
���� ��1��H3PO3������ΪH3PO4��Ag+����ԭΪAg�����ԭ���غ�͵���غ�д����
��2���������һ�����������������������������ĵڶ������룻
��3����ϡ��Һ�Т�H3PO3��aq��?H2PO3-��aq��+H+��aq����H=akJ/mol
��H2PO3-��aq��?HPO32-��aq��+H+��aq����H=bkJ/mol
��H+��aq��+OH-��aq��=H2O��l����H=ckJ/mol
��˹���ɼ����+��-2�۵õ���
��4��NaH2PO3ˮ�ⷴӦΪ��H2PO3-+H2O?H3PO3+OH-��Kh=$\frac{c��{H}_{3}P{O}_{3}��c��O{H}^{-}��}{c��{H}_{2}P{{O}_{3}}^{-}��}$=$\frac{c��{H}_{3}P{O}_{3}��c��O{H}^{-}��}{c��{H}_{2}P{{O}_{3}}^{-}��}$��$\frac{c��{H}^{+}��}{c��{H}^{+}��}$=$\frac{Kw}{K{a}_{1}}$��ƽ�ⳣ��ֻ���¶ȱ仯��
��5��aΪ��Դ������bΪ��Դ������AΪ�����ң�BΪ�����ң���Ʒ���з���2H++HPO32-=H3PO3��Ӧ�õ������ᣬ��ϵ����غ���������������Ƶ�������
��� �⣺��1��H3PO3������ΪH3PO4��Ag+����ԭΪAg����Ӧ�����ӷ���ʽΪ��H3PO3+2Ag++H2O=H3PO4+2Ag+2H+��
�ʴ�Ϊ��H3PO3+2Ag++H2O=H3PO4+2Ag+2H+��
��2������ƽ���ƶ��ĽǶȽ��ͣ��������һ�����������������������������ĵڶ������룬���Ե�һ�������Ka1Զ���ڵڶ��������Ka2��
�ʴ�Ϊ���������һ�����������������������������ĵڶ������룬���Ե�һ�������Ka1Զ���ڵڶ��������Ka2��
��3����ϡ��Һ�Т�H3PO3��aq��?H2PO3-��aq��+H+��aq����H=akJ/mol
��H2PO3-��aq��?HPO32-��aq��+H+��aq����H=bkJ/mol
��H+��aq��+OH-��aq��=H2O��l����H=ckJ/mol
��˹���ɼ����+��-2�۵õ���H3PO3��aq��+2NaOH��aq��?Na2HPO3��aq��+2H2O��l����H=��a+b+2c��KJ/mol��
�ʴ�Ϊ��a+b+2c��
��4��NaH2PO3ˮ�ⷴӦΪ��H2PO3-+H2O?H3PO3+OH-��Kh=$\frac{c��{H}_{3}P{O}_{3}��c��O{H}^{-}��}{c��{H}_{2}P{{O}_{3}}^{-}��}$=$\frac{c��{H}_{3}P{O}_{3}��c��O{H}^{-}��}{c��{H}_{2}P{{O}_{3}}^{-}��}$��$\frac{c��{H}^{+}��}{c��{H}^{+}��}$=$\frac{Kw}{K{a}_{1}}$��Ka1��Kh=Kw������Kh=$\frac{1{0}^{-14}}{5��1{0}^{-2}}$=2��10-13����������I2��������������ⷴӦ����Һ�ļ��Ա�����$\frac{c��{H}_{3}P{O}_{3}��c��O{H}^{-}��}{c��{H}_{2}P{{O}_{3}}^{-}��}$���ֲ��䣬����$\frac{c��{H}_{3}P{O}_{3}��}{c��{H}_{2}P{{O}_{3}}^{-}��}$������
�ʴ�Ϊ��2��10-13������
��5����aΪ��Դ������bΪ��Դ������AΪ�����ң�BΪ�����ң���Ʒ���з���2H++HPO32-=H3PO3��Ӧ�õ������ᣬ
�ʴ�Ϊ������2H++HPO32-=H3PO3��
�ڵõ�0.1mol������ת��0.2mol���ӣ�����0.2molNaOH������=0.2mol��40g/mol=8g��
�ʴ�Ϊ��8��
���� ���⿼����������ʵ���ƽ��Ӱ�����ء��Ȼ�ѧ����ʽ��д��˹���ɼ��㡢����ˮ���ˮ��ƽ�ⳣ�����㡢ԭ�������͵���ԭ��Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ��ͬ�¶��£��������Ȼ�������ֱ������ͬ����Ģ�����ˮ����0.1 mol•L-1���ᡢ��0.1 mol•L-1�Ȼ�þ��Һ����0.1 mol•L-1��������Һ�У�Ag+Ũ�ȣ��٣���=�ڣ��� | |
| B�� | ��NH4HCO3��AlCl3��FeCl2��KAl��SO4��2����Һ�ֱ�������ɡ����գ������ܵõ�ԭ���� | |
| C�� | ��ֱ�Ӹ���Ksp����ֵ��С�Ƚ���������ˮ�е��ܽ�ȴ�С | |
| D�� | 25��ʱ��Ksp��AgCl����Ksp��AgI������AgCl������Һ�м���KI���壬�л�ɫ�������� |
���������ϡ���������������500��ʱ�������������ȫ�ֽ⣬�ֽ�����к�����������������������ˮ�����ȣ�
��ʵ��̽����ij��ѧС��ѡ��ͼ1��ʾ����װ�ý���ʵ�飨���ּг�װ���ԣ�

��1����֤�ֽ�����к��а�����ˮ��������̽����������ɷ֣�
����ѡ��װ�õ���ȷ����˳��ΪACBD����װ�õ���ĸ��ţ���
��A�й�����ȫ�ֽ���Ϊ����ɫ��ĩ�����ʵ��֤��A�в��������ΪFe2O3�������� FeO �� Fe3O4��ȡ����A�в��������������ϡ����ʹ����ȫ�ܽ⣬����Һ�еμ��������Ը��������Һ�������������Һ����ɫ�������������Fe2O3������FeO��Fe3O4��
��2��̽���ֽ�����е������������װ��A-E-F-B����ʵ�飮
��ʵ������й۲쵽��E��û����������F����Һ��ɫ���ݴ˵ó��Ľ����Ƿֽ��������SO2��û��SO3��
��ʵ��֤����NH4��2Fe��SO4��2���ȷֽ�����������⣬����N2���ɣ�д��A�з�Ӧ�Ļ�ѧ����ʽ2��NH4��2Fe��SO4��2$\frac{\underline{\;500��C\;}}{\;}$Fe2O3+4SO2��+2NH3��+N2��+5H2O����
��3����֪BaSO3��BaSO4��������ˮ����ѧС����������ʵ��������SO2���壬����������Ա�����Һ��Ӧ��̽��ʵ�飨ͼ2����
| ��� | ���� | ���� |
| I | ��ͼ2װ���ڳ�SO2���� | G�У�������ð����������ɫ������H�У�������ð����������ɫ������Һ���Ϸ������Ժ���ɫ��������ʧ |
| �� | ��G��H�й��˳���ɫ�������ֱ����ϡ������ | G��H�еİ�ɫ���������ܽ� |
�ڹ���G���а�ɫ����������ԭ������Ϊ����������װ���е������μ��˷�Ӧ��
��д��H�з�����Ӧ�����ӷ���ʽ3SO2+3Ba2++2NO3-+2H2O=3BaSO4��+2NO��+4H+��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
| 1 | �� | |||||||
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | �� | ||
��1���ǽ�������ǿ��Ԫ����F����Ԫ�ط��ţ���
��2��д��������γ�ԭ�Ӹ�����Ϊ1��1������ĵ���ʽ��
 ���û����������������������ط�Ӧ�����ӷ���ʽ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
���û����������������������ط�Ӧ�����ӷ���ʽ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����3������������Ӧ��ˮ�����м�����ǿ����NaOH���ѧʽ����д����������ߵ�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
��4���ɢ٢ڢ�����Ԫ���е�������ɵ�һ��ǿ�ᣬ��ǿ���ϡ��Һ��ͭ��Ӧ�����ӷ���ʽ3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O
������������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ�еı仯������A��һ�ֵ���ɫ���壬������������X��һ���⻯�������ΪҺ�壮��

��1��A��Һ��X��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2H2O=4Na++4OH-+O2����
��2������Y��һ�ִ�����Ⱦ���ɫ���д̼�����ζ�����壬ֱ���ŷŻ��γ����꣮д������Y����ˮ��Ӧ�����ӷ���ʽ��SO2+Cl2+2H2O�T4H++2Cl-+SO42-��
��3����100mL 18mol/L��FŨ��Һ�м������ͭƬ������ʹ֮��ַ�Ӧ��������������Ϊ11.2L������£�����Ӧ������ת�Ƶĵ�����ΪNA ��6.02��1023���á�NA����ʾ��
| A�� | 6�� | B�� | 8�� | C�� | 10�� | D�� | 14�� |
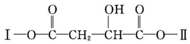 �����Т�Ϊδ֪���ֵĽṹ����
�����Т�Ϊδ֪���ֵĽṹ����


 ��
�� ֱ��ԼΪ9.3 nm�Ľ�������������й�˵������ȷ����( )
ֱ��ԼΪ9.3 nm�Ľ�������������й�˵������ȷ����( )