��Ŀ����
16�����������ʣ���N2 ��Na2O2 ��NaOH ��HCl ��H2O2 ��Na2S ��NH4Cl��1��ֻ�����Ӽ����ɵ������Ǣ�
��2��ֻ�ɼ��Լ����ɵ������Ǣ�
��3��ֻ�ɷǼ��Լ����ɵ������Ǣ�
��4��ֻ�ɷǽ���Ԫ����ɵ����ӻ������Ǣ�
��5���ɼ��Լ��ͷǼ��Լ����ɵ������Ǣ�
��6�������Ӽ��ͼ��Լ����ɵ������Ǣ�
��7�������Ӽ��ͷǼ��Լ����ɵ������Ǣ�
��8��д���٢ڢĵ���ʽ
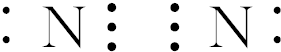 ��
�� ��
�� ��
��
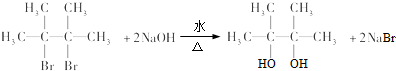
���� һ����˵�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ����������Ӽ��Ļ����������ӻ����ֻ�����ۼ��Ļ������ǹ��ۻ�����ݴ˷������
��� �⣺��N2 ��ֻ���Ǽ��Լ������ڵ��ʣ�
��Na2O2�к������Ӽ��ͷǼ��Լ����������Ӽ������
��NaOH����NH4Cl�к������Ӽ��ͼ��Լ����������Ӽ������
��HCl��ֻ�����Լ������ڹ��ۻ����
��H2O2 ����H-O���Լ���O-O�Ǽ��Լ������ڹ��ۻ����
��Na2S��ֻ�����Ӽ����������ӻ����
�ʴ�Ϊ����1���ޣ�2���ܣ�3���٣�4���ߣ�5���ݣ�6���ۣ�7���ڣ�
��8�����ݵ���ʽ����д�������٢ۢĵ���ʽ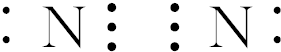 ��
�� ��
�� ��
��
�ʴ�Ϊ��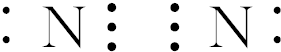 ��
�� ��
�� ��
��
���� ���⿼�������ʺͻ�ѧ���Ĺ�ϵ������ʽ����д����ȷ�����д��ڵĻ�ѧ���ǽⱾ��ؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
18�������йظ�����ʴ�������˵����ȷ���ǣ�������
| A�� | �ֹ���Ǧ���ӣ��ֹܿɱ����� | |
| B�� | ������Ũ�������ۻ����ɱ����ڲ�������ʴ | |
| C�� | ���������������ױ���ʴ | |
| D�� | �����������ⸯʴʱ��������Ӧ��Fe-3e-�TFe3+ |
19�����淴Ӧ2NO2��g��?2NO��g��+02��g���ں����ܱզ����н��У�
�ٵ�λʱ��������n��mol��O2��ͬʱ����2n��mol��NO2
�ڵ�λʱ��������n��mol��O2��ͬʱ����2n��mol��NO
����NO2��NO��O2��ʾ�ķ�Ӧ����֮��Ϊ2��2��1
�ܻ���������ɫ���ٸı�
�ݻ��������ܶȲ��ٸı�
��������ѹǿ���ٸı��״̬
��������ƽ����Է����������ٸı��״̬��
�ٵ�λʱ��������n��mol��O2��ͬʱ����2n��mol��NO2
�ڵ�λʱ��������n��mol��O2��ͬʱ����2n��mol��NO
����NO2��NO��O2��ʾ�ķ�Ӧ����֮��Ϊ2��2��1
�ܻ���������ɫ���ٸı�
�ݻ��������ܶȲ��ٸı�
��������ѹǿ���ٸı��״̬
��������ƽ����Է����������ٸı��״̬��
| A�� | �٢ܢޢ� | B�� | �ڢݢޢ� | C�� | �٢ۢܢ� | D�� | ȫ�� |
11��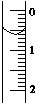 ��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����
��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����
��ʵ�鲽��
��1������ʽ�ζ��ܣ����������ƣ���ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�����������ƣ��ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.100 0mol•L-1 NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ0.60mL��
��4���ζ�������Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫʱ��
ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
��ʵ���¼
�����ݴ���������
��1����ʵ���������ݣ��ɵ�c�����۰״ף�=0.75mol•L-1����ʽ���㣩�����۰״�������=4.5g•100mL-1����ʽ���㣩��
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����ab��д��ţ���
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦����
 ��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����
��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1������ʵ�鲽��
��1������ʽ�ζ��ܣ����������ƣ���ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�����������ƣ��ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.100 0mol•L-1 NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ0.60mL��
��4���ζ�������Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫʱ��
ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
��ʵ���¼
| �ζ�����ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
��1����ʵ���������ݣ��ɵ�c�����۰״ף�=0.75mol•L-1����ʽ���㣩�����۰״�������=4.5g•100mL-1����ʽ���㣩��
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����ab��д��ţ���
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦����
5��������Ԫ��X��Y��M��N��ԭ���������ε��������ǵĺ˵����֮��Ϊ32��ԭ������������֮��Ϊ10��X��Mͬ���壬Y��Nͬ���壬X��Mԭ�ӵ�����������֮�͵���Yԭ�ӵĴ�����������������������ȷ���ǣ�������
| A�� | ��Y��N������������У�Y��N����ԭ��֮���Ϊ˫�� | |
| B�� | һ�������£�Y�������û���N���ʣ�M���û���X���� | |
| C�� | NԪ��λ��Ԫ�����ڱ��е�3����I V�� | |
| D�� | ����Ԫ�ص�ԭ�Ӱ뾶��X��Y��M��N |
6��ij��������̼���⡢������Ԫ����ɣ���������ͼ��C-H����O-H����C-O���������գ��˴Ź���������ʾ�÷�������4�����շ棬����л���Ľṹ��ʽ�ǣ�������
| A�� | CH3CH��OH��CH3 | B�� | CH3CH2CH2CH2OH | C�� | CH3CH2CH2OH | D�� | CH3CH2OCH2CH3 |
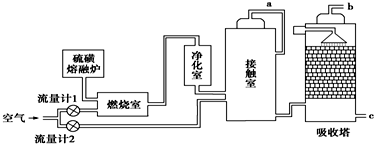
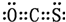 ��̼ԭ�Ӻ�����ӵĹ������ʽΪ
��̼ԭ�Ӻ�����ӵĹ������ʽΪ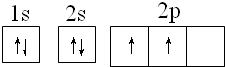 ��
��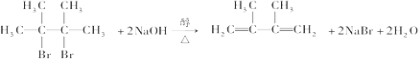 ��
�� ��E���Ҷ����Ĺ�ϵ��ͬϵ�
��E���Ҷ����Ĺ�ϵ��ͬϵ� ��
��