��Ŀ����
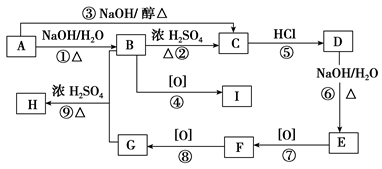
�л���A��B��C��D��E��F��G��H�ת����ϵ����ͼ��ʾ��5.2 g F����100 mL 1 mol/L NaOH��Һǡ����ȫ�кͣ�0.1 mol F����������NaHCO3��Ӧ���ڱ�״���·ų�4.48 L CO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���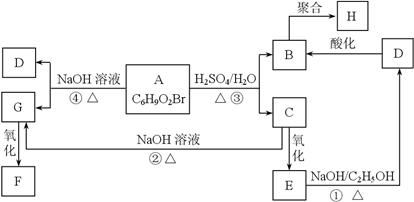
��1��д������C�еĹ����ŵ����ƣ� ��
��2��д������F��H�Ľṹ��ʽ��
F ��H ��
��3��д����Ӧ�١��ܵĻ�ѧ��Ӧ���ͣ��� ���� ��
��4��д���仯�١��۵Ļ�ѧ����ʽ��
��
��
��5��д����Է���������B��14������B������ͬ�����ŵ��������ʵĽṹʽ�������������칹����
��
��1���ǻ�����ԭ��
���������������ת����ϵ��֪AΪ����ˮ������B��C����C��E��D��B��֪B��C̼ԭ������ȣ�B�ܷ����Ӿ۷�Ӧ������D��B��֪DӦ����̼̼˫�����Ȼ���ӦΪCH2=CHCOOH��CӦ�����ǻ�����ԭ�ӣ�0.1mol F����������NaHCO3��Ӧ���ڱ�״���·ų�4.48L CO2��˵��F�к���2���Ȼ�����C��G��F��֪CӦΪBrCH2CH2CH2OH����EΪBrCH2CH2COOH��DΪCH2=CHCOONa��AΪBrCH2CH2CH2OOCCH=CH2��GΪHOCH2CH2CH2OH��FΪHOOCCH2COOH��HΪ ��
��
�����Ϸ�����֪CΪBrCH2CH2CH2OH�������ǻ�����ԭ�ӣ��ʴ�Ϊ���ǻ�����ԭ�ӣ�
��2�������Ϸ�����֪FΪHOOCCH2COOH��HΪ ��
��
�ʴ�Ϊ��HOOCCH2COOH�� ��
��
��3��EΪBrCH2CH2COOH��DΪCH2=CHCOONa��E����DΪ��ȥ��Ӧ����Ϊ����ˮ�ⷴӦ��
�ʴ�Ϊ����ȥ��Ӧ��ˮ�ⷴӦ��
��4����Ӧ�ٵĻ�ѧ����ʽΪ ��Ӧ�۵ķ���ʽΪ
��Ӧ�۵ķ���ʽΪ
��5����Է���������B��14������B������ͬ�����ŵ��������ʵĽṹʽ��CH3CH=CHCOOH��CH2=CHCH2COOH��
���㣺�����л���ϳɵ����֪ʶ��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д���֪���Ҵ��ɱ�ǿ����������Ϊ���ᡣBrCH2CH=CHCH2Br�ɾ�������Ӧ��ȡHOOCCH��Cl��CH2COOH��������Ӧ������������
| A��ˮ�ⷴӦ���ӳɷ�Ӧ��������Ӧ | B���ӳɷ�Ӧ��ˮ�ⷴӦ��������Ӧ |
| C��ˮ�ⷴӦ��������Ӧ���ӳɷ�Ӧ | D���ӳɷ�Ӧ��������Ӧ��ˮ�ⷴӦ |
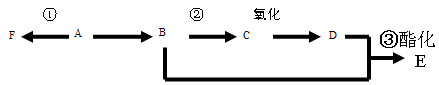
��14�֣�������һ����Ҫ�Ļ���ԭ�ϣ��������Ա���Ϊԭ�ϣ�������˾ƥ�֡����Ϻ߷��ӻ�����ĺϳ���·ͼ��
��֪��1��
��2��
��3��R��OH��HBr R��Br��H2O
R��Br��H2O
��1��д����Ӧ���ͣ���Ӧ������_____________��Ӧ����Ӧ������________________��Ӧ��
��2��д�������л���Ľṹ��ʽ��E___________��G_____________��F____________��
��3�����Լ��鰢˾ƥ����Ʒ�к���ˮ������Լ���__________����д��ĸ����
| A��̼��������Һ | B���Ȼ�����Һ | C����ɫʯ����Һ | D������������ͭ����Һ |
��15�֣�������ң�G�����ڱ��������ҩ����ʹЧ�����ڲ���ҡ���ͼ�ǰ�����ҵ�һ���ϳ�·�ߡ�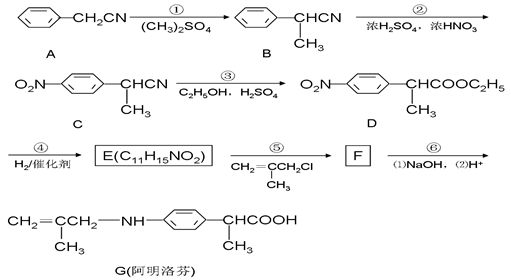
��1��E�Ľṹ��ʽΪ ���ڵķ�Ӧ���ͣ� ��
��2��D�к��������ŵ�����Ϊ �� ��
��3����Ӧ�ۿ��Կ�����������Ӧ���ܷ�Ӧ����һ�����������CN������ȫˮ�ⷴӦ�����Ȼ�����COOH������д���ڶ�����Ӧ�Ļ�ѧ����ʽ�� ��
��4������д�����ַ�������������E��ͬ���칹��Ľṹ��ʽ �� ��
������FeCl3��Һ������ɫ��Ӧ ���ܷ���ˮ�ⷴӦ�������ܷ���������Ӧ
�۱����ϵ�ȡ����ֻ��2�֣��ұ����ϵĺ˴Ź����������������շ�
��5�������йذ�����ң�G����˵����ȷ���ǣ� ��
| A������ʽΪC13H17NO2 | B�����ڰ����� |
| C���������ᷴӦ������ | D���ܷ���ȡ�����Ӿۡ���������ԭ��Ӧ |




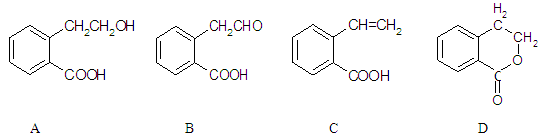
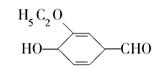 )��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ����Ũ����
)��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ����Ũ����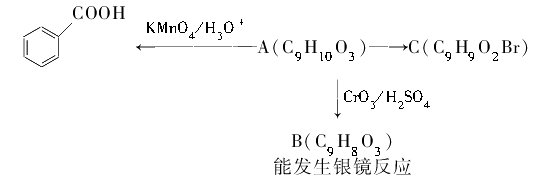 ��ʾ��
��ʾ�� b���뱽��ֱ��������̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ�
b���뱽��ֱ��������̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ�