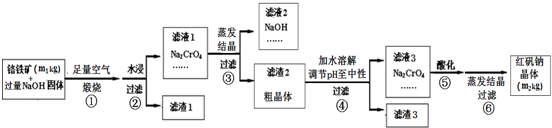
��Ŀ����
Ԫ��A��B��C��D��E��Fλ��Ԫ�����ڱ���ǰ�����ڣ�ԭ���������������������Ϣ���£�
��ش�
��1��EԪ�������ڱ��е�λ�� ���� �壬F�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
��2��A��B�γɵ�16���ӷ����к� ���м����ԱȽ�B��C��DԪ�ص縺���ɴ�С��˳�� ����Ԫ�ط��ţ���
��3���ԱȽ�B��D�������ʵ��۵�ߵ�B D�����=����
��4����֪B���ʵ�ȼ����Ϊ393.5kJmol-1��BC��ȼ����Ϊ110.5kJmol-1����д��BC2��B���ʷ�Ӧ���Ȼ�ѧ��Ӧ����ʽ���û�ѧ�����ʾ�� ��
| Ԫ�� | �����Ϣ |
| A | ��������Ȼ������������� |
| B | ԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��� |
| C | �ǵؿ��к�����ߵ�Ԫ�� |
| D | ��BԪ��ͬ���壬�ؿ��к���������C |
| E | ��ɫ��dz��ɫ |
| F | ��������������бز����ٵ�Ԫ��֮һ |
��1��EԪ�������ڱ��е�λ��
��2��A��B�γɵ�16���ӷ����к�
��3���ԱȽ�B��D�������ʵ��۵�ߵ�B
��4����֪B���ʵ�ȼ����Ϊ393.5kJmol-1��BC��ȼ����Ϊ110.5kJmol-1����д��BC2��B���ʷ�Ӧ���Ȼ�ѧ��Ӧ����ʽ���û�ѧ�����ʾ��
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������A��������Ȼ������������壬��AΪHԪ�أ�Bԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��ӣ���B��ԭ������Ϊ6��ΪCԪ�أ�C�ǵؿ��к�����ߵ�Ԫ�أ���CΪOԪ�أ�D��BԪ��ͬ���壬�ؿ��к���������C����DΪSiԪ�أ�EԪ�ص���ɫ��dz��ɫ����EΪKԪ�أ�F��������������бز����ٵ�Ԫ��֮һ����FΪFeԪ�أ��ݴ˽��н��
���
�⣺A��������Ȼ������������壬��AΪHԪ�أ�Bԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��ӣ���B��ԭ������Ϊ6��ΪCԪ�أ�C�ǵؿ��к�����ߵ�Ԫ�أ���CΪOԪ�أ�D��BԪ��ͬ���壬�ؿ��к���������C����DΪSiԪ�أ�EԪ�ص���ɫ��dz��ɫ����EΪKԪ�أ�F��������������бز����ٵ�Ԫ��֮һ����FΪFeԪ�أ�
��1���ɷ�����֪��EΪKԪ�أ���ԭ������Ϊ19��λ�����ڱ��е�������IA�壻FΪFeԪ�أ���ԭ������Ϊ26��Fe�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p64s2��
�ʴ�Ϊ���ģ�IA��1s22s22p63s23p64s2��
��2��AΪH��BΪCԪ�أ�A��B�γɵ�16���ӷ���Ϊ��ϩ����ϩ�����к���һ��̼̼˫����̼̼˫���к���1���м���B��C��D�ֱ�ΪC��O��SiԪ�أ�Ԫ�طǽ�����Խǿ���縺��Խ��������Ԫ�ص縺���ɴ�С��˳��Ϊ��O��C��Si��
�ʴ�Ϊ��1��O��C��Si��
��3��B��D�ֱ�ΪC��SiԪ�أ����߳�������Ϊ���ʯ������裬���ھ�����̼̼�������϶̣�����ʯ���۵���ھ���裬����B��D��
�ʴ�Ϊ������
��4��BΪC��CΪO����֪C���ʵ�ȼ����Ϊ393.5kJmol-1�����Ȼ�ѧ����ʽΪ����O2��g��+C��s��=2CO2��g����H=-393.5kJ/mol��
CO��ȼ����Ϊ110.5kJmol-1�����Ȼ�ѧ����ʽΪ����CO��g��+
O2��g��=CO2��g����H=-110.5kJ/mol��
���ݸ�˹���ɣ���-�ڡ�2�ɵ�CO2��C���ʷ�Ӧ���Ȼ�ѧ��Ӧ����ʽ��CO2��g��+C��s��=2CO��g����H=-172.5kJ/mol��
�ʴ�Ϊ��CO2��g��+C��s��=2CO��g����H=-172.5kJ/mol��
��1���ɷ�����֪��EΪKԪ�أ���ԭ������Ϊ19��λ�����ڱ��е�������IA�壻FΪFeԪ�أ���ԭ������Ϊ26��Fe�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p64s2��
�ʴ�Ϊ���ģ�IA��1s22s22p63s23p64s2��
��2��AΪH��BΪCԪ�أ�A��B�γɵ�16���ӷ���Ϊ��ϩ����ϩ�����к���һ��̼̼˫����̼̼˫���к���1���м���B��C��D�ֱ�ΪC��O��SiԪ�أ�Ԫ�طǽ�����Խǿ���縺��Խ��������Ԫ�ص縺���ɴ�С��˳��Ϊ��O��C��Si��
�ʴ�Ϊ��1��O��C��Si��
��3��B��D�ֱ�ΪC��SiԪ�أ����߳�������Ϊ���ʯ������裬���ھ�����̼̼�������϶̣�����ʯ���۵���ھ���裬����B��D��
�ʴ�Ϊ������
��4��BΪC��CΪO����֪C���ʵ�ȼ����Ϊ393.5kJmol-1�����Ȼ�ѧ����ʽΪ����O2��g��+C��s��=2CO2��g����H=-393.5kJ/mol��
CO��ȼ����Ϊ110.5kJmol-1�����Ȼ�ѧ����ʽΪ����CO��g��+
| 1 |
| 2 |
���ݸ�˹���ɣ���-�ڡ�2�ɵ�CO2��C���ʷ�Ӧ���Ȼ�ѧ��Ӧ����ʽ��CO2��g��+C��s��=2CO��g����H=-172.5kJ/mol��
�ʴ�Ϊ��CO2��g��+C��s��=2CO��g����H=-172.5kJ/mol��
���������⿼��λ�á��ṹ�����ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ������漰�������ʡ��縺�Դ�С�Ƚϡ��Ȼ�ѧ����ʽ����д��֪ʶ�����������Ϣ��ȷ�ƶϸ�Ԫ������Ϊ���ؼ���ע������Ԫ�����ڱ��ṹ��Ԫ�����������ݣ�

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
�Ѻ������������й�ÿ��Ҫ����5�ڶ����ҵ�����ʯ��ռ���纣������ʯó������һ�����ϣ�����ȫ������ʯ�۸�����ǣ��й�������ҵЭ����Ĵ����DZغͱ��ع�˾̸��������������������ʯ˵����ȷ���ǣ�������
| A�����������Ҫ�ɷ���Fe3O4 |
| B������ʯ����Ҫ�ɷ����������Ҫ�ɷ���ͬ |
| C���������ĩ�����������KSCN��Һ����Һ���ɫ |
| D��FeO�׳����� |
����ˮ�����߲ˡ�����Ʒ�и�����ά����C���������ԵĿ�˥�����ã����ױ�����������ij����С�����õ�ζ�����ij��֭��ά����C�ĺ������仯ѧ����ʽ���£�����˵����ȷ���ǣ�������


| A��������ӦΪȡ����Ӧ |
| B��ά����C������������ˮ��ֻ�õ�1�ֲ��� |
| C������C���������� |
| D������ά����C�ķ���ʽΪC6H6O6 |
����ʵ�������ȷ���ǣ�������
| A����pH��ֽ���ij������ˮ��pHֵΪ3.5 |
| B����ⷨ����ͭ������ͭ�ӵ�Դ������ |
| C�����Ȼ���������������ˮ�У������Ȼ�����Һ |
| D�����Ȼ�����Һ�������ɣ������գ��͵õ��Ȼ������� |
���й����л����˵������ȷ���ǣ�������
| A��������ϩ����ϩ��Ϊͬ���칹�� |
| B����ά�ء����ά�������л��߷��ӻ����� |
| C�����顢��ϩ�ͱ����ɷ����ӳɷ�Ӧ |
| D��������ʳ��ƾ����˵��ۡ������ǡ��Ҵ��Ȼ�ѧ�仯���� |
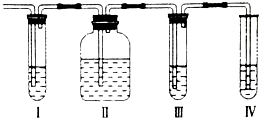 ��ʵ��������ȡ����ϩ�г����������Ķ�������ij��ѧ��ȤС���������ͼ��ʾ��ʵ��װ����ȷ����������������Ƿ���SO2��C2H4����ش��������⣺
��ʵ��������ȡ����ϩ�г����������Ķ�������ij��ѧ��ȤС���������ͼ��ʾ��ʵ��װ����ȷ����������������Ƿ���SO2��C2H4����ش��������⣺