��Ŀ����
����������������ʽΪC4H2O4Fe���ṹ��ʽΪ ����һ������ʹ�õ���ǿ������
����һ������ʹ�õ���ǿ������
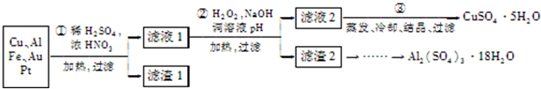
��1����ͼΪʵ����ģ�ҵ��ȡ����������������ͼ��
�ٸ������⣬����д��������Ľṹ��ʽ�� ��
���̷���FeSO4?7H2O���ڱ���������γɵ�������Ҫ�� ���ѧʽ����
�۲���Y���� ����ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
���жϲ���Y�С�ϴ�ӡ�������������Ʒ������ϴ����ʵ�鷽���� ��
��2�����һ��ʵ�鷽����֤�����ø�����������Ʒ���������Σ��ɹ�ѡ�õ��Լ���KSCN��Һ��H2O2��Һ��ϡ���ᣮ������д�±���Ӧ�ո�
 ����һ������ʹ�õ���ǿ������
����һ������ʹ�õ���ǿ��������1����ͼΪʵ����ģ�ҵ��ȡ����������������ͼ��
�ٸ������⣬����д��������Ľṹ��ʽ��
���̷���FeSO4?7H2O���ڱ���������γɵ�������Ҫ��
�۲���Y����
���жϲ���Y�С�ϴ�ӡ�������������Ʒ������ϴ����ʵ�鷽����
��2�����һ��ʵ�鷽����֤�����ø�����������Ʒ���������Σ��ɹ�ѡ�õ��Լ���KSCN��Һ��H2O2��Һ��ϡ���ᣮ������д�±���Ӧ�ո�
| ���� | ʵ����������� |
| �� | ȡ������������Ʒ1.5g������ϡ����25mL����ˮϡ����50mL������ʹ����ȫ�ܽⲢ��Ӧ����ȴ����ˣ���ȥ���ɵĸ����ἰ���ܹ����ķ�Ӧ���������Һ |
| �� | |
| �� |

���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,����ʵ�鷽�������
ר�⣺ʵ�������
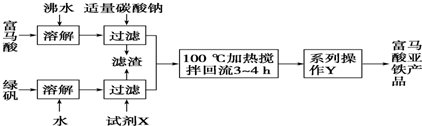
�������������̿�֪����ȡ����������������Ϊ���ȷֱ��ܽ��̷������ᣬȻ����̼�����븻���ᷴӦ���ɸ������ƣ������������̷���Ӧ���ɸ�����������Һ��Ȼ��ͨ������Ũ���õ�������������Ʒ��
��1���ٸ��ݸ�����Ľṹʽ д����ṹ��ʽ��
д����ṹ��ʽ��
�ڸ��������������ױ����������������Ӽ������ӵ�ˮ����з�����
�۵õ��ĺ��и�������������Һ��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��������õ�������������Ʒ��
�ܸ�������������Ŀ���������������Na+��SO42-�ȣ����ԴӼ���ϴ��Һ���Ƿ�Na+��SO42-�����ʵ�飻
��2���ȵμ����軯�ؼ�����Һ�в��������������ӣ������������������������������������ӣ��ټ������軯����Һ����Һ��ɺ�ɫ��֤��ԭ��Һ�д����������ӣ�
��1���ٸ��ݸ�����Ľṹʽ
 д����ṹ��ʽ��
д����ṹ��ʽ���ڸ��������������ױ����������������Ӽ������ӵ�ˮ����з�����
�۵õ��ĺ��и�������������Һ��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��������õ�������������Ʒ��
�ܸ�������������Ŀ���������������Na+��SO42-�ȣ����ԴӼ���ϴ��Һ���Ƿ�Na+��SO42-�����ʵ�飻
��2���ȵμ����軯�ؼ�����Һ�в��������������ӣ������������������������������������ӣ��ټ������軯����Һ����Һ��ɺ�ɫ��֤��ԭ��Һ�д����������ӣ�
���
�⣺��ȡ����������������Ϊ���ȷֱ��ܽ��̷������ᣬȻ����̼�����븻���ᷴӦ���ɸ������ƣ������������̷���Ӧ���ɸ�����������Һ��Ȼ��ͨ������Ũ���õ�������������Ʒ��
��1���ٸ��ݽṹʽ ��֪��������Ľṹ��ʽΪ��HOOCHC�TCHCOOH��
��֪��������Ľṹ��ʽΪ��HOOCHC�TCHCOOH��
�ʴ�Ϊ��HOOCHC�TCHCOOH��
�������������Ӳ��ȶ������ױ����������������ӣ�����������Һ�лᷢ��ˮ�⣬�������������������̷���FeSO4��7H20���ڱ���������γɵ�������Ҫ�����������������������ʣ�
�ʴ�Ϊ��Fe2��SO4��3��Fe��OH��3��
�۵õ��ĺ��и�������������Һ��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��������õ�������������Ʒ��
�ʴ�Ϊ������Ũ����
�ܸ�������������Ŀ���������������Na+��SO42-�ȣ����ԴӼ���ϴ��Һ���Ƿ�Na+��SO42-�����ʵ�飬���Լ��鷽���ǣ�ȡ���һ��ϴ��Һ���ȼ���ϡ���ᣬ�ټ����Ȼ�����Һ���ް�ɫ�������ɣ�˵����ϴ�Ӹɾ���
�ʴ�Ϊ��ȡϴ��Һ�������м������������ữ���ټ����Ȼ�����Һ���ް�ɫ�������ɣ�
��2��������������ʱ�����ж���Һ�в����������ӣ�Ȼ��������������������軯����Һ���������ӣ��Ӷ��ж���Һ���Ƿ����������ӣ���������ķ���Ϊ����ȡ������Һ���μ�KSCN��Һ������Ѫ��ɫ��֤����Һ��û�����������ӣ�
������������Һ�еμ�H2O2��Һ����Һ��Ѫ��ɫ��֤��ԭ��Һ�д����������ӣ�
�ʴ�Ϊ��
��
��1���ٸ��ݽṹʽ
 ��֪��������Ľṹ��ʽΪ��HOOCHC�TCHCOOH��
��֪��������Ľṹ��ʽΪ��HOOCHC�TCHCOOH���ʴ�Ϊ��HOOCHC�TCHCOOH��
�������������Ӳ��ȶ������ױ����������������ӣ�����������Һ�лᷢ��ˮ�⣬�������������������̷���FeSO4��7H20���ڱ���������γɵ�������Ҫ�����������������������ʣ�
�ʴ�Ϊ��Fe2��SO4��3��Fe��OH��3��
�۵õ��ĺ��и�������������Һ��Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��������õ�������������Ʒ��
�ʴ�Ϊ������Ũ����
�ܸ�������������Ŀ���������������Na+��SO42-�ȣ����ԴӼ���ϴ��Һ���Ƿ�Na+��SO42-�����ʵ�飬���Լ��鷽���ǣ�ȡ���һ��ϴ��Һ���ȼ���ϡ���ᣬ�ټ����Ȼ�����Һ���ް�ɫ�������ɣ�˵����ϴ�Ӹɾ���
�ʴ�Ϊ��ȡϴ��Һ�������м������������ữ���ټ����Ȼ�����Һ���ް�ɫ�������ɣ�
��2��������������ʱ�����ж���Һ�в����������ӣ�Ȼ��������������������軯����Һ���������ӣ��Ӷ��ж���Һ���Ƿ����������ӣ���������ķ���Ϊ����ȡ������Һ���μ�KSCN��Һ������Ѫ��ɫ��֤����Һ��û�����������ӣ�
������������Һ�еμ�H2O2��Һ����Һ��Ѫ��ɫ��֤��ԭ��Һ�д����������ӣ�
�ʴ�Ϊ��
| ���� | ʵ����������� |
| �� | |
| �� | ȡ������Һ���μ�KSCN��Һ������Ѫ��ɫ |
| �� | ����������Һ�еμ�H2O2��Һ����Һ��Ѫ��ɫ |
���������⿼����ʵ����ģ�ҵ��ȡ������������������Ŀ�Ѷ��еȣ��漰�������Ʊ��������������ӵļ���֪ʶ����ȷ����Ʊ����̼��Ʊ����������ԭ��Ϊ���ؼ�����������������ѧ���ķ�����������������ѧʵ��������

��ϰ��ϵ�д�
�����Ŀ
�����ǻ�ѧѧϰ���о��ij����ֶΣ����з������ݺͽ��۶���ȷ���ǣ�������
| A��H2O��HCOOH����NH4��2Fe��SO4��2�������������������� |
| B��HCl��H2SO4��HNO3�����������ԣ������������� |
| C����������������ȸʯ���dz���������ʯ |
| D��Na2CO3��Ba��OH��2��NH4Cl��Na2O2���������ӻ����� |
�屶����һ�ֳ������в�ҩ������Ч�ɷ�ΪX����һ��������X�ɷֱ�ת��ΪY��Z������˵��������ǣ�������


| A��1molX�������2molBr2����ȡ����Ӧ |
| B��Y���ӽṹ����2������̼ԭ�� |
| C��Y�ܷ����ӳɡ�ȡ������ȥ�����������۷�Ӧ |
| D��1mol Z�������7molNaOH������Ӧ |