��Ŀ����
���ص���̨������Ԥ���ж�ҪԤ�����еĿ���������Ԥ����һ�㽫���п����е�SO2�����Ϳ���������ĺ���������g/cm3��ʾ����Ϊ8���ȼ���Ŀǰ���ⶨ������SO2����ʱ��Ҫ��һЩ������ԭ��Ӧ��
��֪��SO2����������KMnO4��Һ��Ӧʱ��MnO4-����ԭΪMn2+��SO2������ΪSO42-��
����ҩƷ��0.1mol/L������KMnO4��Һ��������������Ʒ����ֽ��pH��ֽ��������и��⣺
��1������200mL0.1mol/L������KMnO4��Һ���õ������� �� ������������ͷ�ιܡ��ձ��ȣ��������������������� ��
��2���ڲⶨSO2�Ϳ��������ʱ������Ӧ�ⶨ ��ԭ���� ��
��3��д��SO2������KMnO4��Һ��Ӧ�����ӷ���ʽ�� �������������Ϊa cm3/min����t minʱ��200mL 0.1mol/L��������Һǡ����ɫ���������SO2�ĺ���Ϊ ��g/cm3����
��4����Ҫ�ⶨ�����п���������ĺ�������Ҫ����������� ��
��5��������SO2�ĺ��������ü��ܲⶨ����ԭ���ǣ�ָʾ��KIO3�������ã����ܷ����ķ�Ӧ��δ��ƽ���У���KIO3+SO2+H2O��I2+H2SO4+KHSO4 ��KIO3+SO2+H2O��KI+H2SO4
��Ƽ����ܵ�ԭ���ǰ�������������ѧ����ʽ�еķ���ʽ ����١��ڡ�����Ƶģ������� ��
��֪��SO2����������KMnO4��Һ��Ӧʱ��MnO4-����ԭΪMn2+��SO2������ΪSO42-��
����ҩƷ��0.1mol/L������KMnO4��Һ��������������Ʒ����ֽ��pH��ֽ��������и��⣺
��1������200mL0.1mol/L������KMnO4��Һ���õ�������
��2���ڲⶨSO2�Ϳ��������ʱ������Ӧ�ⶨ
��3��д��SO2������KMnO4��Һ��Ӧ�����ӷ���ʽ��
��4����Ҫ�ⶨ�����п���������ĺ�������Ҫ�����������
��5��������SO2�ĺ��������ü��ܲⶨ����ԭ���ǣ�ָʾ��KIO3�������ã����ܷ����ķ�Ӧ��δ��ƽ���У���KIO3+SO2+H2O��I2+H2SO4+KHSO4 ��KIO3+SO2+H2O��KI+H2SO4
��Ƽ����ܵ�ԭ���ǰ�������������ѧ����ʽ�еķ���ʽ
���㣺̽�����ʵ���ɻ�������ʵĺ���,��Һ������
ר�⣺ʵ�������
��������1����������200mL0.1mol/L������KMnO4��Һ�IJ����ж���Ҫ���������ݲ����������ƹ����е����ý��
��2�����ݿ����еĹ��峾�����ױ���Һ���ս����жϣ�
��3������������л�ԭ�ԣ����ױ����Ը��������Һ��������������ӣ��ݴ�д����Ӧ�����ӷ���ʽ�����ݷ�Ӧ����ʽ���������١����������ҺŨ�ȼ���������ж�������ĺ�����
��4��Ҫ�ⶨ�����п���������ĺ�������Ҫ�ⶨ����ǰ�����պ����������������ʢ��������������
��5�����ݷ�Ӧԭ��Ӧ�ñ��ڲ�������Ӧ�����г������Եķ�Ӧ������н��
��2�����ݿ����еĹ��峾�����ױ���Һ���ս����жϣ�
��3������������л�ԭ�ԣ����ױ����Ը��������Һ��������������ӣ��ݴ�д����Ӧ�����ӷ���ʽ�����ݷ�Ӧ����ʽ���������١����������ҺŨ�ȼ���������ж�������ĺ�����
��4��Ҫ�ⶨ�����п���������ĺ�������Ҫ�ⶨ����ǰ�����պ����������������ʢ��������������
��5�����ݷ�Ӧԭ��Ӧ�ñ��ڲ�������Ӧ�����г������Եķ�Ӧ������н��
���
��1������200mL0.1mol?L-1����KMnO4��Һ������ʵ����û��200mL����ƿ��Ӧ��250mL����ƿ���ƣ�����������ƽ����������ع��壬���ձ��м�ˮ�ܽⲢ�ò��������裬��ȴ���ò�����ת��������ƿ�У�����������������������ˮ����Һ��̶���1��2cmʱ���ý�ͷ�ιܵμ����̶��ߣ���ȱ�ٵ�����Ϊ��������ƽ��250mL����ƿ�����������ܽ���������Һ����ʱ���衢�����ܽ�����ã���ת����Һʱ�ò�����������
�ʴ�Ϊ��������ƽ��250mL����ƿ�����裻
��2���ڲⶨSO2�Ϳ��������ʱ�����ڿ����еĹ��峾�����ױ�������Һ���գ���������Ӧ�ⶨ�����������
�ʴ�Ϊ������������������еĹ��峾�����ױ�������Һ���գ�
��3��SO2���л�ԭ�ԣ�������ؾ��������ԣ����߷���������ԭ��Ӧ����SO42-��Mn2+���ӣ���Ӧ�����ӷ���ʽΪ��5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
������ص����ʵ���Ϊ��0.2L��0.1moL/L=0.02moL����t minʱ��ͨ�����������Ϊ��at mL��
���ݷ�Ӧ����ʽ5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��֪��at ml�����к���SO2�����ʵ���Ϊ
��0.02mol=0.05mol��
���������������0.05mol��64g/moL=3.2g��
���Կ�����SO2�ĺ���Ϊ��
g/cm3��
�ʴ�Ϊ��5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
��
��4�������������ԣ������������еĿ�������ֹ������������������ܻ�������Ը��������Һ�У�ʹ��������������Ҫ�ⶨ�����п���������ĺ�����g/L��������Ҫ���������������ǰ�����������������ʢ����������������
�ʴ�Ϊ������ǰ�����պ����������������ʢ��������������
��5����Ӧ���У���Ӧǰ����Һ����ɫ�仯���ԣ�����Ӧ���У���Ӧǰ��û����������������Ƽ����ܵ�ԭ��Ӧ��ѡ��Ӧ�٣�
�ʴ�Ϊ���٣��÷�Ӧǰ����Һ����ɫ�仯����ָʾ�յ㣻
�ʴ�Ϊ��������ƽ��250mL����ƿ�����裻
��2���ڲⶨSO2�Ϳ��������ʱ�����ڿ����еĹ��峾�����ױ�������Һ���գ���������Ӧ�ⶨ�����������
�ʴ�Ϊ������������������еĹ��峾�����ױ�������Һ���գ�
��3��SO2���л�ԭ�ԣ�������ؾ��������ԣ����߷���������ԭ��Ӧ����SO42-��Mn2+���ӣ���Ӧ�����ӷ���ʽΪ��5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
������ص����ʵ���Ϊ��0.2L��0.1moL/L=0.02moL����t minʱ��ͨ�����������Ϊ��at mL��
���ݷ�Ӧ����ʽ5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��֪��at ml�����к���SO2�����ʵ���Ϊ
| 5 |
| 2 |
���������������0.05mol��64g/moL=3.2g��
���Կ�����SO2�ĺ���Ϊ��
| 3.2 |
| at |
�ʴ�Ϊ��5SO2+2MnO4-+2H2O�T5SO42-+2Mn2++4H+��
| 3.2 |
| at |
��4�������������ԣ������������еĿ�������ֹ������������������ܻ�������Ը��������Һ�У�ʹ��������������Ҫ�ⶨ�����п���������ĺ�����g/L��������Ҫ���������������ǰ�����������������ʢ����������������
�ʴ�Ϊ������ǰ�����պ����������������ʢ��������������
��5����Ӧ���У���Ӧǰ����Һ����ɫ�仯���ԣ�����Ӧ���У���Ӧǰ��û����������������Ƽ����ܵ�ԭ��Ӧ��ѡ��Ӧ�٣�
�ʴ�Ϊ���٣��÷�Ӧǰ����Һ����ɫ�仯����ָʾ�յ㣻
���������⿼����̽�������ж�����̼�����ķ���������һ�����ʵ���Ũ�ȵ���Һ����Ŀ�Ѷ��еȣ�ע������̽��������ɻ�������ʺ����ķ�������ȷ����һ��Ũ�ȵ���Һ����������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�
�����Ŀ
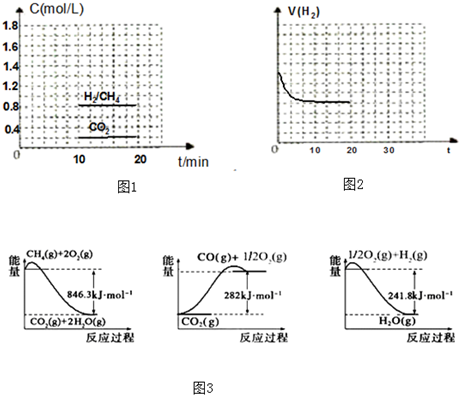
����˵������ȷ���ǣ�������
| A����101kPaʱ��1mol������ȫȼ��ʱ���ų������������������ʵ�ȼ���� |
| B����ͼ���кͷ�Ӧ����1molˮ����ʱ�ķ�Ӧ�Ƚ��к��� |
| C�������ʵ������������������ֱ���ȫȼ�գ����߷ų��������� |
| D���ɵ���Aת��Ϊ����B����H=+119KJ/mol����֪����A�ȵ���B�ȶ� |
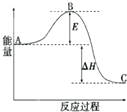 2SO2��g��+O2��g��=2SO3��g����H=-198kJ?mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO2��g��+O2��g��=2SO3��g����H=-198kJ?mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺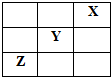 X��Y��Z�Ǣ�����A������ַǽ���Ԫ�أ����������ڱ��е�λ����ͼ��ʾ���Իش�
X��Y��Z�Ǣ�����A������ַǽ���Ԫ�أ����������ڱ��е�λ����ͼ��ʾ���Իش�