��Ŀ����
��ͼ��ʾ��C����������ɫҺ�壬E��X�ǵ��ʣ�E��F��H��Ϊ��ɫ���壮A��B����ͬ��Ԫ����ɣ�
����ͼ����ʾ��ת����ϵ���Իش��������⣺

��1��C�Ľṹʽ�� ��F�ĵ���ʽ�� ����Ӧ�۵ķ�Ӧ������ ��
��2��д�������ʵĻ�ѧʽ��H ��I ��D ��J ��
��3��ʵ��������K��ҺʱҪ���������� ������J��ҺʱҪ���������� ��
��4��д����Ӧ�ڢܵ����ӷ���ʽ��
�� ��
�� ��
����ͼ����ʾ��ת����ϵ���Իش��������⣺

��1��C�Ľṹʽ��
��2��д�������ʵĻ�ѧʽ��H
��3��ʵ��������K��ҺʱҪ����������
��4��д����Ӧ�ڢܵ����ӷ���ʽ��
��
��
���㣺������ƶ�
ר�⣺�ƶ���
��������ͼ��ʾ��C����������ɫҺ�壬E��X�ǵ��ʣ�E��F��H��Ϊ��ɫ���壮B������������Һ��Ӧ��������FΪ������˵��BΪ��Σ�CΪˮ��FΪ������E��Ӧ����C��Ϊˮ����H�������ж�EΪO2��HΪNO��DΪNO2������A�ֽ���������������������ˮ�����������ᣬA��B����ͬ��Ԫ����ɣ�����BΪNH4NO3��GΪBa��NO3��2��XΪ�����ʣ���������Ӧ����KΪ����������������Ӧ����JΪ������������ת����ϵ�����ش�ѡ�����⣮
���
�⣺��ͼ��ʾ��C����������ɫҺ�壬E��X�ǵ��ʣ�E��F��H��Ϊ��ɫ���壮B������������Һ��Ӧ��������FΪ������˵��BΪ��Σ�CΪˮ��FΪ������E��Ӧ����C��Ϊˮ����H�������ж�EΪO2��HΪNO��DΪNO2������A�ֽ���������������������ˮ�����������ᣬA��B����ͬ��Ԫ����ɣ�����BΪNH4NO3��GΪBa��NO3��2��XΪ�����ʣ���������Ӧ����KΪ����������������Ӧ����JΪ��������
��1��CΪˮ��ˮ���ӵĽṹʽΪ��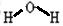 ��FΪNH3�������ĵ���ʽΪ��
��FΪNH3�������ĵ���ʽΪ��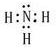 ����Ӧ����Ba��NO3��2+H2SO4��Ũ��=BaSO4��+2HNO3�����ڸ��ֽⷴӦ��
����Ӧ����Ba��NO3��2+H2SO4��Ũ��=BaSO4��+2HNO3�����ڸ��ֽⷴӦ��
�ʴ�Ϊ��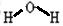 ��
��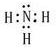 �����ֽⷴӦ��
�����ֽⷴӦ��
��2�����ʵĻ�ѧʽHΪNO��IΪBaSO4��DΪNO2��JΪ��Fe��NO3��2���ʴ�Ϊ��NO��BaSO4��NO2��Fe��NO3��2��
��3��ʵ��������KΪFe��NO3��3��ҺʱҪ����������ϡ�������������ӵ�ˮ�⣬����JΪFe��NO3��2��ҺʱҪ�������������۷�ֹ�������ӱ�����������ϡ�������� �������ӵ�ˮ�⣬
�ʴ�Ϊ��ϡ������ۺ�ϡ���
��4����Ӧ��������Ũ������ȷ����ķ�Ӧ����Ӧ�����ӷ���ʽΪ��Fe+6H++3NO3-
Fe3++3NO2��+3H2O����Ӧ����������������Ӧ�������������ķ�Ӧ����Ӧ�����ӷ���ʽΪ��2Fe3++Fe=3Fe2+��
�ʴ�Ϊ��Fe+6H++3NO3-
Fe3++3NO2��+3H2O��2Fe3++Fe=3Fe2+��
��1��CΪˮ��ˮ���ӵĽṹʽΪ��
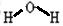 ��FΪNH3�������ĵ���ʽΪ��
��FΪNH3�������ĵ���ʽΪ��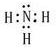 ����Ӧ����Ba��NO3��2+H2SO4��Ũ��=BaSO4��+2HNO3�����ڸ��ֽⷴӦ��
����Ӧ����Ba��NO3��2+H2SO4��Ũ��=BaSO4��+2HNO3�����ڸ��ֽⷴӦ���ʴ�Ϊ��
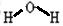 ��
��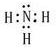 �����ֽⷴӦ��
�����ֽⷴӦ����2�����ʵĻ�ѧʽHΪNO��IΪBaSO4��DΪNO2��JΪ��Fe��NO3��2���ʴ�Ϊ��NO��BaSO4��NO2��Fe��NO3��2��
��3��ʵ��������KΪFe��NO3��3��ҺʱҪ����������ϡ�������������ӵ�ˮ�⣬����JΪFe��NO3��2��ҺʱҪ�������������۷�ֹ�������ӱ�����������ϡ�������� �������ӵ�ˮ�⣬
�ʴ�Ϊ��ϡ������ۺ�ϡ���
��4����Ӧ��������Ũ������ȷ����ķ�Ӧ����Ӧ�����ӷ���ʽΪ��Fe+6H++3NO3-
| ||
�ʴ�Ϊ��Fe+6H++3NO3-
| ||
���������⿼��������ת����ϵ�ķ����ƶϣ��������ʵ��ۺ�Ӧ�ã�����ͼ�ķ����ƶ���C����������ɫҺ�壬E��X�ǵ��ʣ�E��F��H��Ϊ��ɫ���壮A��B����ͬ��Ԫ������ǽ���ؼ��������ںͼӦ���ɵ������ǰ�������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
���з�Ӧ�У�����������ԭ��Ӧ����ˮ�Ȳ������������ֲ��ǻ�ԭ�����ǣ�������
| A��SO3+H2O=H2SO4 |
| B��2Na2O2+2H2O=4NaOH+O2�� |
| C��2F2+2H2O=4HF+O2 |
| D��Mg+2H2O=Mg��OH��2+H2�� |
���з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A��CuSO4��Һ����H2S���壺Cu2++H2S��CuS��+2H+ | ||
B��Ca��HCO3��2��Һ��������NaOH��Һ��ϣ�Ca2++2HC
| ||
| C������������Һ�м����������ữ�Ĺ���������Һ��Fe2++2H++H2O2��Fe2++2H2O | ||
| D�����۵⻯����Һ�ڿ����б�����4I-+O2+2H2O��2I2+4OH- |
��100mL0.1mol/L�Ĵ�����Һ�У���ʹ����ĵ���̶�����������Ũ�ȼ�С���ɲ��õķ����ǣ�������
| A������ |
| B������lmol/L�Ĵ�����Һl00mL |
| C������������0.5mol/L������ |
| D������������lmol/L��NaOH��Һ |
�������ӷ���ʽ��д��ȷ���ǣ�������
| A��̼�����Ƶ�ˮ�ⷴӦ��HCO3-+H2O�TCO32-+H3O+ | ||||
| B����������Һ�еμӹ�����ˮ��Ag++2NH3?H2O=[Ag��NH3��2]++2H2O | ||||
C����ͭ���缫�������ͭ��Һ��2Cu2++2H2O
| ||||
| D��������[KAl��SO4��2]��Һ����μ���Ba��OH��2��Һ��SO42-ǡ����ȫ������2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� |



