��Ŀ����
��ҵ�Ƽ�����ʳ��ˮ��ͨ�백���Ͷ�����̼�����̼�����ƾ��壬���ķ�Ӧԭ���������л�ѧ����ʽ��ʾ��
NH3��CO2��H2O��NH4HCO3������
NH4HCO3��NaCl(����)��HaHCO3����NH4Cl������
������̼�����ƾ�����ȷֽ�ɵõ����
��ش�
(1)��ҵ���ƴ����г������������Ȼ������ʣ���ԭ����________��
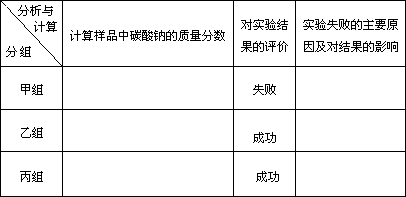
(2)���мס��ҡ�������С���ѧ�������ⶨij��ҵ������Ʒ��Na2CO3�������������ֱ���Ʋ����ʵ�����£�
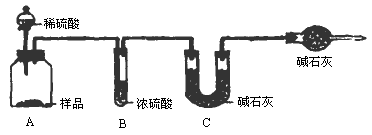
���飺ȡ10.00g��Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C�м�ʯ�ҵ�����Ϊ3.52g��
���飺ȡ10.00g��Ʒ�����1000mL��Һ���ü�ʽ�ζ�����ȡ25.00mL������ƿ�У����������ָʾ������0.150mol��L��1�ı�������Һ�ζ����յ�(�йط�ӦΪNa2CO3��2HCl��2NaCl��2H2O��CO2��)���������ƽ��ʵ����������������ƽ��ֵΪ30.00mL��
���飺ȡ10.00g��Ʒ�������м�����������ᣬֱ����Ʒ��������ð�����������������ﲢ�ڸ���������ȴ�����º�������������ȡ���ȴ��������ֱ���������Ĺ���������������Ϊֹ����ʱ���ù��������Ϊ10.99g�������������������

������
|
����(1)NaHCO3�ǴӺ����� ����(2)����,84.8��,ʧ�ܵ�ԭ�������ɵ�CO2û�б�ȫ�����գ���һ���������ڷ�Ӧ�����У����²ⶨ���ƫ�ͣ�;����,95.4��;����,95.4�� |

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| |||||||||||
| |||||||||||




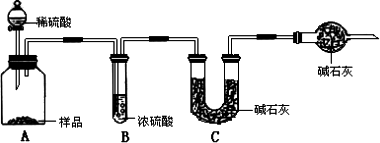
 Na2CO3+CO2��+H2O��ij������ȤС��ͬѧ�����ա������Ƽ��ԭ�����������ͼ��ʾ��һ��ʵ��װ�ã�
Na2CO3+CO2��+H2O��ij������ȤС��ͬѧ�����ա������Ƽ��ԭ�����������ͼ��ʾ��һ��ʵ��װ�ã� 