��Ŀ����
����˵��������ǣ�������
| A��0.1 mol?L-1�Ĵ����м���ˮ�������������ʹƽ������뷽���ƶ� |
| B����pH�Ĵ����̼�ᣬ�ֱ��ˮϡ�ͺ���Һ��pH����ȣ�������м���ˮ������� |
| C�������ʵ���Ũ�ȵ�CH3COONa��Na2CO3��Һ��������Һ��ˮ�ĵ���̶ȴ� |
| D��������10ml 0.02 mol?L-1HCl��Һ��10ml 0.02 mol?L-1Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20ml������Һ��pH=10 |
���㣺���������ˮ��Һ�еĵ���ƽ��,����ˮ���Ӧ��
ר�⣺
������A������Ӱ�����ƽ������ط�����
B��PH��ͬʱ������Խ�������Ũ��Խ��
C����Һ������Ũ����ͬ����Խ�������ε����ˮ��̶�Խ�ݴ˽��
D�������Ϻ��c��OH-�����ټ�����Һ��pH��
B��PH��ͬʱ������Խ�������Ũ��Խ��
C����Һ������Ũ����ͬ����Խ�������ε����ˮ��̶�Խ�ݴ˽��
D�������Ϻ��c��OH-�����ټ�����Һ��pH��
���
�⣺A����ˮϡ�ʹٽ�����ĵ��룬�ӱ������ʹ����ĵ���ƽ�����ƣ���A��ȷ��
B��pH��ͬʱ������Խ�������Ũ��Խ�����Ե�pH�Ĵ����̼�ᣬ���ԣ����̼�ᣬ�ֱ��ˮϡ�ͺ�pH����ȣ�������м���ˮ��������٣���B��ȷ��
C�������ʵ���Ũ�ȵ�CH3COONa��Na2CO3��Һ��ˮ���Լ��Դٽ�ˮ�ĵ��룬�������Ӷ�Ӧ����Խ���������ˮ��̶�Խ�������Ϸ������ԣ����̼�����������Na2CO3��Һ��ˮ��̶ȱȴ�����ǿ��ˮ�ĵ���̶ȴ�C��ȷ��
D��10ml0.02mol/L��HCl��Һ��10ml0.02mol/L��Ba��OH��2��Һ��ֻ�Ϻ�����Ϻ���Һ���Ϊ20ml����Ӧ�����Һ��c��OH-��=
=0.01mol/L��c��H+��=
mol/L=1��10-12mol/L��pH=-lg1��10-12=12����D����
��ѡD��
B��pH��ͬʱ������Խ�������Ũ��Խ�����Ե�pH�Ĵ����̼�ᣬ���ԣ����̼�ᣬ�ֱ��ˮϡ�ͺ�pH����ȣ�������м���ˮ��������٣���B��ȷ��
C�������ʵ���Ũ�ȵ�CH3COONa��Na2CO3��Һ��ˮ���Լ��Դٽ�ˮ�ĵ��룬�������Ӷ�Ӧ����Խ���������ˮ��̶�Խ�������Ϸ������ԣ����̼�����������Na2CO3��Һ��ˮ��̶ȱȴ�����ǿ��ˮ�ĵ���̶ȴ�C��ȷ��
D��10ml0.02mol/L��HCl��Һ��10ml0.02mol/L��Ba��OH��2��Һ��ֻ�Ϻ�����Ϻ���Һ���Ϊ20ml����Ӧ�����Һ��c��OH-��=
| 0.04mol/L��0.01L-0.02mol/L��0.01L |
| 0.02L |
| 1��10-14 |
| 0.01 |
��ѡD��
���������⿼��������ĵ���ƽ�⼰Ӱ�����أ��ε�ˮ��ԭ����Ӧ�õȣ�����ע����ҺpH�ļ��㷽������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ
�ɶ�����Ԫ�صļ������γɵ�ij�����һ�������Ӻ�һ�������Ӻ��������֮��Ϊ20������˵������ȷ���ǣ�������
| A�������������Ӻ������Ӹ�����һ����� |
| B��������һ�������Ӽ��������й��ۼ� |
| C������Ԫ��һ������ͬһ���ڣ�Ҳ����ͬһ���� |
| D�������������Ӱ뾶һ�����������Ӱ뾶 |
���г����µ�������Һ�����±�����
�����й���������ȷ���ǣ�������
| �� | �� | �� | �� | |
| ��Һ | ��ˮ | ����������Һ | ���� | ���� |
| pH | 11 | 11 | 3 | 3 |
| A���ֱ��ˮϡ��10����������Һ��pH���ڣ��٣��ܣ��� |
| B�����ۡ��ֱܷ�ϡ�͵�pH=5����ˮ����������ӵ�Ũ�Ⱦ���С100�� |
| C���ڢ١����зֱ�����������Ȼ�茶���ٵ�pH��С���ڵ�pH���� |
| D�����١���������Һ�������ϣ�������Һ�У�c��OH-����c��H+����1 |
 �ռ�ʵ���ҡ��칬һ�š��Ĺ���ϵͳ������������ȼ�ϵ�أ�RFC����RFC��һ�ֽ�ˮ��⼼��������ȼ�ϵ�ؼ������ϵĿɳ���أ���ͼΪRFC����ԭ��ʾ��ͼ���й�˵����ȷ���ǣ�������
�ռ�ʵ���ҡ��칬һ�š��Ĺ���ϵͳ������������ȼ�ϵ�أ�RFC����RFC��һ�ֽ�ˮ��⼼��������ȼ�ϵ�ؼ������ϵĿɳ���أ���ͼΪRFC����ԭ��ʾ��ͼ���й�˵����ȷ���ǣ�������| A��b���Ϸ����ĵ缫��Ӧ�ǣ�4H2O+4e-�T2H2��+4OH- |
| B��d���Ϸ����ĵ缫��Ӧ�ǣ�O2+4H++4e-�T2H2O |
| C��c���Ͻ��л�ԭ��Ӧ��B�е�H+��ͨ����Ĥ����A |
| D������0.1 mol����ת��ʱ��a������1.12 L O2����״���£� |
��ѧ�ҽ��������Ƴ�һ������ϸ��ȼ�ϵ�أ�����ϸ�����л���ת��Ϊ���飬Ȼ����ͨ����KOHΪ����ʵ�ȼ�ϵ�ط��磮��ظ�����ӦΪ��������
| A��CH4-8e-+8OH-=CO2+6H2O |
| B��O2+4H++4e-=2H2O |
| C��CH4+10OH--8e-=C32-+7H2O |
| D��O2+2H2O+4e-=4OH- |
�������ƺͶ��ȷӣ� ���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
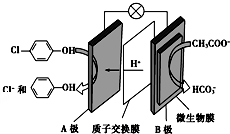
 ���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
| A��B�������� |
| B��B�������� |
| C��ÿת��2mol���ӣ���1molCH3COO-������ |
D��A���缫��ӦʽΪ��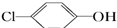 +2e-+H+ +2e-+H+ +Cl- +Cl- |
 ��Ȫʵ����һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ��Ը�����ͼ���ش��������⣺
��Ȫʵ����һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ��Ը�����ͼ���ش��������⣺