��Ŀ����
11������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ���������������������ų��������ȣ���֪0.2 molҺ̬����������Һ̬˫��ˮ��Ӧʱ�ų�128kJ����������1����д���÷�Ӧ���Ȼ�ѧ����ʽ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640kJ/mol��
��2���÷�Ӧ�б���ԭ����OԪ�أ���Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
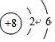 ����Ԫ��ͨ��ת��Ϊ������Σ�笠����ӵĵ���ʽΪ
����Ԫ��ͨ��ת��Ϊ������Σ�笠����ӵĵ���ʽΪ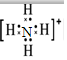 ��
��
���� ��1��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȣ��������������ϻ�ѧ����ʽ�����Ӧ�����ʱ䣬��ע���ʾۼ�״̬д���Ȼ�ѧ����ʽ��
��2�����ݻ��ϼ۱仯�жϱ���ԭ��Ԫ�أ�Ȼ���ϸ�Ԫ��ԭ�ӵ�ԭ������д����ԭ�ӽṹʾ��ͼ��笠�����Ϊ�����ӣ���Ҫ���������ɼ��������ӣ�
��� �⣺��1��0.2 molҺ̬����������Һ̬˫��ˮ��Ӧʱ�ų�128kJ��������1molҺ̬�º������ⷴӦ���ȣ�128kJ��$\frac{1mol}{0.2mol}$=640kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��l��+2H2O2��l��=N2��g��+4 H2O��g����H=-640kJ/mol
�ʴ�Ϊ��N2H4��l��+2 H2O2��l��=N2��g��+4H2O��g����H=-640kJ/mol��
��2����ӦN2H4��l��+2H2O2��l��=N2��g��+4H2O��g���У�N2H4��-2��NԪ�ػ��ϼ����߱�������H2O2��-1��OԪ�ػ��ϼ۽��ͱ���ԭ�����е�Ԫ��ΪO��Oԭ�ӵ�ԭ������Ϊ8����ԭ�ӽṹʾ��ͼΪ��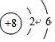 ��笠�����Ϊ���������ӣ�����ʽ����Ҫ���������ɼ�ԭ�ӵ��������ӣ������ʽΪ
��笠�����Ϊ���������ӣ�����ʽ����Ҫ���������ɼ�ԭ�ӵ��������ӣ������ʽΪ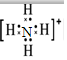 ��
��
�ʴ�Ϊ��O��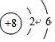 ��
��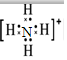 ��
��
���� ���⿼���˷�Ӧ�����ʱ��Ӧ�ã���Ŀ�Ѷ��еȣ��漰�Ȼ�ѧ����ʽ��д��������ԭ��Ӧ������ʽ��ԭ�ӽṹʾ��ͼ��֪ʶ����ȷ�Ȼ�ѧ����ʽ����дԭ��Ϊ���ؼ���ע�����ճ�����ѧ����ĸ����ʾ����������������ѧ���Ĺ淶����������

 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף���1���ñ���������Һ�ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��
��2�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���D��
A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
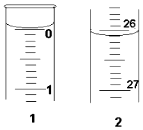
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����������
��Һ�����Ϊ26.10mL��
��4��ijѧ����������ʵ��ֱ��¼�й����������
| �ζ� ���� | �������������� Һ�����/mL | 0.1000mol•L+1����������mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 |
| ������ | 25.00 | 0.22 | 26.31 |
��1���������Թ���ȼ�ϵ�أ����ط�Ӧԭ��Ϊ4NH3+3O2=2N2+6H2O��NH3Ӧͨ��ȼ�ϵ�صĸ������������������������֪�������ҺΪKOH��Һ�������ĵ缫��ӦʽΪ2NH3+6OH--6e-�TN2+6H2O��
��2����0.5L�����ܱ������У�һ������N2��H2���з�Ӧ��N2��g��+3H2��g��?2NH3��g����H=bkJ•mol-1���仯ѧƽ�ⳣ��K���¶ȵĹ�ϵ�����
| �¶�/�� | 200 | 300 | 400 |
| K | 1.0 | 0.85 | 0.5 |
�������ϣ�Ϊ������ƽ��ʱH2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��ad������ĸ��ţ���
a������ѹǿ b��ʹ�ú��ʵĴ���
c�������¶� d����ʱ����������е�NH3
��400��ʱ�����ijʱ�̰��������������������ʵ����ֱ�Ϊ3mol��2mol��1molʱ����ʱ�̸÷�Ӧ��v����N2�� С��v�棨N2��������ڡ���С�ڡ����ڡ�����
��3����֪��
��4NH3��g��+3O2��g���T2N2��g��+6H2O��g����H=-1266.8kJ•mol-1
��N2��g��+O2��g���T2NO��g����H=+180.5kJ•mol-1
д�������´��������Ȼ�ѧ����ʽ4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8 kJ/mol��
| A�� | ͭ���ڷ����ڳ�ʪ�Ŀ������������⣬����Cu��OH��2 | |
| B�� | ͭ˿��������ȼ������CuCl2������Ӧ����Cu2S��˵��������Cl2��S | |
| C�� | CuO����Cu2O�ȶ�������������CuO���Էֽ�����Cu2O������ | |
| D�� | ��ɫ��CuSO4•5H2O�������ȷֽ�ת��Ϊ��ɫ��CuSO4 |
 ��ͼ��ʾ����A��E�������ʵ��ת����ϵ������AΪ����ɫ���壬BΪ���ʣ�
��ͼ��ʾ����A��E�������ʵ��ת����ϵ������AΪ����ɫ���壬BΪ���ʣ� 


