��Ŀ����
Ϊ��Ԥ����ȱ�������ҹ��������涨ÿǧ�˵�����Ӧ����40��50���˵ĵ���أ�
��1��Ϊ����ijʳ�����Ƿ��е���أ�ijͬѧȡʳ����Ʒ428g�ܽ���������������ữ�ĵ��۵⻯����Һ����Һ����ɫ���÷�Ӧ�����ӷ���ʽΪ�� �����������뻹ԭ������������ǣ� ��
��2��Ϊ�˽�һ��ȷ����Ʒ�Ƿ�Ϊ�ϸ��Ʒ����������0.030mol?L-1�������������Һ18.00mLǡ�ðѲ�����I2��Ӧ�꣨I2+2S2O32-=2I-+S4O62-������ͨ�������жϸõ����Ƿ�Ϊ�ϸ��Ʒ��Ҫд��������̣���
��1��Ϊ����ijʳ�����Ƿ��е���أ�ijͬѧȡʳ����Ʒ428g�ܽ���������������ữ�ĵ��۵⻯����Һ����Һ����ɫ���÷�Ӧ�����ӷ���ʽΪ��
��2��Ϊ�˽�һ��ȷ����Ʒ�Ƿ�Ϊ�ϸ��Ʒ����������0.030mol?L-1�������������Һ18.00mLǡ�ðѲ�����I2��Ӧ�꣨I2+2S2O32-=2I-+S4O62-������ͨ�������жϸõ����Ƿ�Ϊ�ϸ��Ʒ��Ҫд��������̣���
���㣺������ԭ��Ӧ�ļ���,��ѧ����ʽ���йؼ���
ר�⣺������
��������1��KIO3�������ữ�ĵ���KI��Һ����������ԭ��Ӧ���ɵⵥ�ʣ������۱��������Ԫ�صĻ��ϼ۱仯��������ԭ��Ӧ����������㣻
��2������������Ӧ�Ļ�ѧ����ʽ�������ҳ���ϵʽ��KIO3----3I2----6Na2S2O3����KIO3-6Na2S2O3����϶�Ӧ���ʵ����������ʵ����Ĺ�ϵ�����⣮
��2������������Ӧ�Ļ�ѧ����ʽ�������ҳ���ϵʽ��KIO3----3I2----6Na2S2O3����KIO3-6Na2S2O3����϶�Ӧ���ʵ����������ʵ����Ĺ�ϵ�����⣮
���
�⣺��1��KIO3�������ữ�ĵ���KI��Һ����������ԭ��Ӧ���ɵⵥ�ʣ������۱��������ӷ�ӦΪ5I-+IO3-+6H+=3I2+3H2O����IԪ�صĻ��ϼ۱仯��֪��5molI-ʧȥ������1molIO3-ԭ�ӵõ����ӵ���Ŀ��ȣ����������뻹ԭ�����Ϊ�ⵥ�ʣ���������Ϊ5��1���ʴ�Ϊ��5I-+IO3-+6H+=3I2+3H2O��5��1��
��2��n ��Na2S2O3��=0.030 mol/L��0.018 L=0.00054 mol��
����������Ӧ�Ļ�ѧ����ʽ�������ҳ���ϵʽ��KIO3----3I2----6Na2S2O3����KIO3-6Na2S2O3��
�����ص�������m
KIO3-------------------6Na2S2O3
214g 6 mol
m 0.00054 mol
214g��6mol=m ��KIO3����0.00054 mol
m��KIO3��=
=0.01926 g=19.26 mg��
��ÿǧ��ʳ��������KIO3������Ϊ
=45 mg����40 mg��50 mg��Χ�ڣ����Ըüӵ�ʳ���Ǻϸ��Ʒ��
����40 mg��50 mg��Χ�ڣ����Ըüӵ�ʳ���Ǻϸ��Ʒ��
��2��n ��Na2S2O3��=0.030 mol/L��0.018 L=0.00054 mol��
����������Ӧ�Ļ�ѧ����ʽ�������ҳ���ϵʽ��KIO3----3I2----6Na2S2O3����KIO3-6Na2S2O3��
�����ص�������m
KIO3-------------------6Na2S2O3
214g 6 mol
m 0.00054 mol
214g��6mol=m ��KIO3����0.00054 mol
m��KIO3��=
| 0.00054mol��214g |
| 6mol |
��ÿǧ��ʳ��������KIO3������Ϊ
| 19.26mg |
| 0.428kg |
����40 mg��50 mg��Χ�ڣ����Ըüӵ�ʳ���Ǻϸ��Ʒ��
���������⿼��������ԭ��Ӧ�ļ��㣬��Ŀ�ѶȲ�������ѧ���ķ��������ͼ��������Ŀ��飬��2�����жϵ���غ���������ƵĹ�ϵʽ�ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����ʵ��װ�ã��̶�װ����ȥ���Ͳ�����ȷ���ǣ�
A�� ֤���¶ȶ�ƽ���ƶ���Ӱ�� |
B�� ����HCl� |
C�� �Ʊ����������� |
D�� ��֤��������ȥ��Ӧ��������ϩ |
���б仯���������仯���ǣ�������
| A�������ڷŵ������±�ɳ��� |
| B���øɱ����� |
| C��Ư�IJ�ñ�����ڿ����б�� |
| D�����ȵ�������ˮ����ͭ |

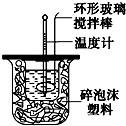 ��ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
��ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�