��Ŀ����
���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺������Ҫ��ش��������⣺

��1����̬Ni�ļ۵��ӹ��ͣ������Ų�ʽ��Ϊ ��
��2��Ni��CO��4����ΪҺ̬��������CCl4�������л��ܼ�����Ni��CO��4���� ���壮
��3��Ni2+���붡��ͪ뿣� �����������Ⱥ�ɫ��������A��
�����������Ⱥ�ɫ��������A��
�ٶ���ͪ뿷�����̼ԭ�ӵ��ӻ���������� ��1mol�÷����к��е�̼̼�Ҽ���̼���Ҽ�������Ϊ ��
�ڰ�����ˮ�е��ܽ��Զ���ڼ��飬��ԭ���� ��
���Ⱥ�ɫ��������A�У��ṹ���ң�������һ�㹲�ۼ��⣬��������λ�������������ͼ��1�����λ���������
����ʾ��Ni2+����λ��Ϊ4����λ���á�������ʾ������á�������ʾ��
��4���ݱ�����ij�ֺ���þ������̼����Ԫ�صľ�����г����ԣ������ͳ��������һ�������Ľṹ��ͼ2��ʾ����þ���Ļ�ѧʽΪ ��

��1����̬Ni�ļ۵��ӹ��ͣ������Ų�ʽ��Ϊ
��2��Ni��CO��4����ΪҺ̬��������CCl4�������л��ܼ�����Ni��CO��4����
��3��Ni2+���붡��ͪ뿣�
 �����������Ⱥ�ɫ��������A��
�����������Ⱥ�ɫ��������A���ٶ���ͪ뿷�����̼ԭ�ӵ��ӻ����������
�ڰ�����ˮ�е��ܽ��Զ���ڼ��飬��ԭ����
���Ⱥ�ɫ��������A�У��ṹ���ң�������һ�㹲�ۼ��⣬��������λ�������������ͼ��1�����λ���������
����ʾ��Ni2+����λ��Ϊ4����λ���á�������ʾ������á�������ʾ��
��4���ݱ�����ij�ֺ���þ������̼����Ԫ�صľ�����г����ԣ������ͳ��������һ�������Ľṹ��ͼ2��ʾ����þ���Ļ�ѧʽΪ
���㣺�����ijɼ����,ԭ�Ӻ�������Ų�,���Ӿ���,�����ļ���,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺
��������1������28��Ԫ�أ���ԭ�Ӻ�����28�����ӣ�3d��4s����Ϊ��۵��ӣ����ݹ���ԭ����д��۵����Ų�ʽ��
��2�����ݷ��Ӿ�����������ʵ�ͨ�Է�����
��3���ڶ���ͪ���̼ԭ�Ӽ��е�������˫��������ӻ�������sp2��sp3�ӻ���C-CΪ̼̼�Ҽ���C=N����һ��̼���Ҽ���
������Ĵ���Ӱ�����ʵ��۷е���ܽ��ԣ�
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ԭ������ԭ��֮���γ������
��4�����þ�̯��ȷ�������Ļ�ѧʽ��λ�ڶ����ϵ�һ��ԭ�ӱ�8������ռ�У�λ�������ϵ�ԭ�ӱ�2������ռ�У�λ�������ϵ�һ��ԭ�ӱ�һ������ռ�У��ݴ˼��㾧���Ļ�ѧʽ��
��2�����ݷ��Ӿ�����������ʵ�ͨ�Է�����
��3���ڶ���ͪ���̼ԭ�Ӽ��е�������˫��������ӻ�������sp2��sp3�ӻ���C-CΪ̼̼�Ҽ���C=N����һ��̼���Ҽ���
������Ĵ���Ӱ�����ʵ��۷е���ܽ��ԣ�
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ԭ������ԭ��֮���γ������
��4�����þ�̯��ȷ�������Ļ�ѧʽ��λ�ڶ����ϵ�һ��ԭ�ӱ�8������ռ�У�λ�������ϵ�ԭ�ӱ�2������ռ�У�λ�������ϵ�һ��ԭ�ӱ�һ������ռ�У��ݴ˼��㾧���Ļ�ѧʽ��
���
�⣺��1����������Ų�Ϊ[Ar]3d84s2����۵����Ų�ʽ��3d84s2���ʴ�Ϊ��3d84s2��
��2�����Ӿ�����۷е�ϵͣ���֪Ni��CO��4����ΪҺ̬��������CCl4�������л��ܼ�����Ni��CO��4���ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��3���ٶ���ͪ���̼ԭ�Ӽ��е�������˫����̼̼�����ϵ�̼ԭ�ӵ��ӻ�������sp2��̼̼˫���ϵ�̼ԭ�ӵ��ӻ�������sp3�ӻ���C-CΪ̼̼�Ҽ���C=N����һ��̼���Ҽ�����1mol�÷����к��е�̼̼�Ҽ���̼���Ҽ�������Ϊ5NA��
�ʴ�Ϊ��sp2sp3��5NA��
��N��O��Fԭ�����γ����������Ĵ���Ӱ�����ʵ��۷е���ܽ��ԣ�����NH3��H2O���Ӽ������������������H2O���Ӽ䲻������������������ܽ��Դ�
�ʴ�Ϊ����������ˮ���Ӽ��γ��������������Ӳ��ܣ�
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ͬ��������ԭ������ԭ��֮���γ��������ͼ��ʾ��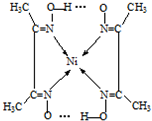 ��
��
�ʴ�Ϊ�� ��
��
��4��̼ԭ��λ�ڸþ����������ϣ����Ըþ����к���һ��̼ԭ�ӣ�þԭ�Ӹ���=
��8�����Ըþ�������1��þԭ�ӣ���ԭ�Ӹ���=
��6���þ����к���3����ԭ�ӣ����Ըþ����Ļ�ѧʽΪMgNi3C��
�ʴ�Ϊ��MgNi3C��
��2�����Ӿ�����۷е�ϵͣ���֪Ni��CO��4����ΪҺ̬��������CCl4�������л��ܼ�����Ni��CO��4���ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��3���ٶ���ͪ���̼ԭ�Ӽ��е�������˫����̼̼�����ϵ�̼ԭ�ӵ��ӻ�������sp2��̼̼˫���ϵ�̼ԭ�ӵ��ӻ�������sp3�ӻ���C-CΪ̼̼�Ҽ���C=N����һ��̼���Ҽ�����1mol�÷����к��е�̼̼�Ҽ���̼���Ҽ�������Ϊ5NA��
�ʴ�Ϊ��sp2sp3��5NA��
��N��O��Fԭ�����γ����������Ĵ���Ӱ�����ʵ��۷е���ܽ��ԣ�����NH3��H2O���Ӽ������������������H2O���Ӽ䲻������������������ܽ��Դ�
�ʴ�Ϊ����������ˮ���Ӽ��γ��������������Ӳ��ܣ�
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ͬ��������ԭ������ԭ��֮���γ��������ͼ��ʾ��
 ��
���ʴ�Ϊ��
 ��
����4��̼ԭ��λ�ڸþ����������ϣ����Ըþ����к���һ��̼ԭ�ӣ�þԭ�Ӹ���=
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��MgNi3C��
���������⿼�龧��Ľṹ�����ʵ����ʵ����ϵ��Ӧ�ã�����ʱע������Ų�ʽ����д��������������ӻ����͵��жϷ����Լ��������йؼ��㣬ע��ѧϰ���й����ⷽ���Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
���ھ��������˵����ȷ���ǣ�������
| A���ھ�����ֻҪ�������ӣ���һ���������� |
| B���ھ�����ֻҪ�������ӣ���һ���������� |
| C��ԭ�Ӿ�����۵�һ���Ƚ�������ĸ� |
| D�����Ӿ�����۵�һ���Ƚ�������ĸ� |
�й�NA˵����ȷ���ǣ�������
| A��22.4L��SO3�к���NA����ԭ�� |
| B�����³�ѹ�£�18g��ˮ���еĵ�����ΪNA |
| C��500mL 1mol/L Fe2��SO4��3��Һ��ͨ250mL 3mol/L ��Na2SO4��Һ������������ӵ����ʵ���Ũ��֮��Ϊ1��1 |
| D��������Fe��1mol������ַ�Ӧ����ת�Ƶ�����Ϊ2NA |
����˵����ȷ���ǣ�������
| A�������Ƽ���ŵ��Ǽ����˶��豸�ĸ�ʴ |
| B����ҵ��������ʱ����98%��������������������Ա����γ���������������� |
| C���ϳɰ�����Ȼ�̵��ķ���֮һ |
| D��̼�����׳�С�մ�����һ�������Դ |
��NA��ʾ�����ӵ����������������в���ȷ���ǣ�������
| A��16 g O2��O3�����������������Ϊ8NA |
| B����״���£�2.24 L CH3OH�����й��õ��Ӷ���Ϊ0.5NA |
| C��6 g SiO2�����й������ۼ���Ϊ0.4NA |
| D��0.1 mol 13C18O������������Ϊ1.7NA |

