��Ŀ����
ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨNaOH����������ԼΪ82.0%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol/L��������еζ����Իش��������⣺
��1����ȡ5.0g���������ƹ�����Ʒ�����500mL��Һ���ã�
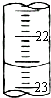
��2����������װ��25.00mL�� �ζ����У�����Һ��λ���ڡ�0���̶����£�����¼�¿̶ȣ�
��3��ȡ20.00mL����Һ������ʵ�����ʹ�õ���Ҫ������ ���÷�̪��ָʾ��ʱ���ζ�����Һ��ɫ�� �պ��� ɫʱΪֹ��
��4���ζ����յ����������ȥ20.00mL������NaOH����������Ϊ ��
��5���Է����ζ���������������Щʵ��������� ��ѡ���ţ���
A����1���У�ת�ƴ���Һ������ƿʱ��δϴ���ձ�
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ����
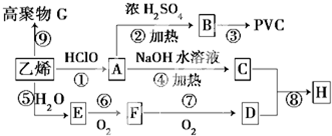
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦��
D���ζ����յ�ʱ���ζ��ܼ�������Һ��
E�����ζ��ܿ�ʼʱ���ӣ����յ�ʱ���ӣ�
��1����ȡ5.0g���������ƹ�����Ʒ�����500mL��Һ���ã�
��2����������װ��25.00mL��
��3��ȡ20.00mL����Һ������ʵ�����ʹ�õ���Ҫ������
��4���ζ����յ����������ȥ20.00mL������NaOH����������Ϊ
��5���Է����ζ���������������Щʵ���������
A����1���У�ת�ƴ���Һ������ƿʱ��δϴ���ձ�
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ����
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦��
D���ζ����յ�ʱ���ζ��ܼ�������Һ��
E�����ζ��ܿ�ʼʱ���ӣ����յ�ʱ���ӣ�
���㣺�к͵ζ�
ר�⣺ʵ����
��������2������Ӧ����ʽ�ζ�����ȡ��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܻ���Һ�ܣ��ü�����ָʾ��ʱ���ζ�ǰ�����������ƣ��ζ��������ɫ��ֹͣ�ζ���
��4��c��HCl��=0.2mol/L��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�������c��NaOH������һ������500mL��Һ��NaOH�����ʵ�����������Ʒ������5.0g��������������
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ������٣�
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫ�ͣ�������ƫ��
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦����NaOH�����ʵ������٣�
D���ζ����յ�ʱ���ζ��ܼ�������Һ�Σ���������ƫ��
E������ʽ�ζ��ܿ�ʼʱ���ӣ�����ƫ�����յ�ʱ���ӣ�����ƫС������֮��ƫС��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܻ���Һ�ܣ��ü�����ָʾ��ʱ���ζ�ǰ�����������ƣ��ζ��������ɫ��ֹͣ�ζ���
��4��c��HCl��=0.2mol/L��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�������c��NaOH������һ������500mL��Һ��NaOH�����ʵ�����������Ʒ������5.0g��������������
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ������٣�
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫ�ͣ�������ƫ��
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦����NaOH�����ʵ������٣�
D���ζ����յ�ʱ���ζ��ܼ�������Һ�Σ���������ƫ��
E������ʽ�ζ��ܿ�ʼʱ���ӣ�����ƫ�����յ�ʱ���ӣ�����ƫС������֮��ƫС��
���
�⣺��2������Ӧ����ʽ�ζ�����ȡ���ʴ�Ϊ����ʽ��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܣ�������ƿ�У��÷�̪��ָʾ��ʱ���ζ�ǰ����̪�����죬����ζ��յ�ʱ�۲쵽��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ����ʽ�ζ��ܡ���ƿ����ɫ���ޣ�
��4��c��HCl��=0.2mol/L��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�����֪c��NaOH��=0.2mol/L��
��500mL��Һ��NaOH�����ʵ���Ϊ0.5L��0.2mol/L=0.1mol����NaOH������Ϊ0.1mol��40g/mol=4.0g������5.0g��Ʒ��NaOH����������Ϊ
��100%=80.0%��
�ʴ�Ϊ��80.0%��
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ������٣���������٣�����ƫ�ͣ���A��ȷ��
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫ�ͣ�������ƫ���ƫ��B����
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦����NaOH�����ʵ������٣���������٣�����ƫ�ͣ���C��ȷ��
D���ζ����յ�ʱ���ζ��ܼ�������Һ�Σ���������ƫ���ƫ��D����
E������ʽ�ζ��ܿ�ʼʱ���ӣ�����ƫ�����յ�ʱ���ӣ�����ƫС������֮��ƫС����������٣�����ƫ�ͣ���E��ȷ��
�ʴ�Ϊ��ACE��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܣ�������ƿ�У��÷�̪��ָʾ��ʱ���ζ�ǰ����̪�����죬����ζ��յ�ʱ�۲쵽��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ����ʽ�ζ��ܡ���ƿ����ɫ���ޣ�
��4��c��HCl��=0.2mol/L��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�����֪c��NaOH��=0.2mol/L��
��500mL��Һ��NaOH�����ʵ���Ϊ0.5L��0.2mol/L=0.1mol����NaOH������Ϊ0.1mol��40g/mol=4.0g������5.0g��Ʒ��NaOH����������Ϊ
| 4.0g |
| 5.0g |
�ʴ�Ϊ��80.0%��
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ������٣���������٣�����ƫ�ͣ���A��ȷ��
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫ�ͣ�������ƫ���ƫ��B����
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦����NaOH�����ʵ������٣���������٣�����ƫ�ͣ���C��ȷ��
D���ζ����յ�ʱ���ζ��ܼ�������Һ�Σ���������ƫ���ƫ��D����
E������ʽ�ζ��ܿ�ʼʱ���ӣ�����ƫ�����յ�ʱ���ӣ�����ƫС������֮��ƫС����������٣�����ƫ�ͣ���E��ȷ��
�ʴ�Ϊ��ACE��
���������⿼���к͵ζ���������Ŀ�Ѷ��еȣ�ע�����ʵ������������ܻ�����������IJ���������ע�����ʵ�����Ҫ���ע�����

��ϰ��ϵ�д�
�����Ŀ
��NAΪ�����ӵ���������ֵ������˵����ȷ���ǣ�������
| A�������£�23g NO2����NA����ԭ�� | ||
| B��0.1mol?L-1���ռ���Һ�к���0.1NA��Na + | ||
| C��22.4 L��CO������1 mol N2�����ķ�������� | ||
D��9 g
|
��X��Y��Z��W��M���ֶ�����Ԫ�أ�����X��Y��Z��Wͬ���ڣ�Z��Mͬ���壻X+��M2-������ͬ�ĵ��Ӳ�ṹ�����Ӱ뾶��Z2-��W-��Y�ĵ��ʾ����۵�ߡ�Ӳ�ȴ���һ����Ҫ�İ뵼����ϣ�����˵����ȷ���ǣ�������
| A��X��M��ԭ�����γ���������������Ŀ֮�ȶ�Ϊ1��2�����ӻ����� |
| B������W��Z��MԪ�ص��⻯����Է����������μ�С��������е����ν��� |
| C��Ԫ��Y��Z��W�ĵ��ʾ�������ͬ�����͵ľ��� |
| D��Ԫ��W��M���ж��ֵ��� |
����ҽ����ʾ��θ�������ˣ����˶��ƻ������Ϊƻ��֭��pH��������
| A������7 | B������7 |
| C��С��7 | D����ȷ�� |
 Ϊ�˲ⶨijNaOH��Ʒ��NaOH���������������ʲ����뷴Ӧ����ij��ѧ��ȤС�����������ʵ�飺
Ϊ�˲ⶨijNaOH��Ʒ��NaOH���������������ʲ����뷴Ӧ����ij��ѧ��ȤС�����������ʵ�飺