��Ŀ����
17�� ���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������£�
���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������£�| ���� | ������������ | ��ѹ/V |
| �� | �ס�Cu | +0.78 |
| �� | Cu���� | -0.15 |
| �� | ����Cu | +1.35 |
| �� | ����Cu | +0.30 |
| A�� | �����ֽ����б��Ļ�ԭ����ǿ | |
| B�� | �������ܴ�����ͭ��Һ���û���ͭ | |
| C�� | �ס������γ�ԭ���ʱ����Ϊ���� | |
| D�� | �ס����γɺϽ�ʱ�����úϽ�¶���ڿ����У����ȱ���ʴ |
���� ����ԭ����е��ӵ������жϽ�����ǿ������ѹֵԽ�����Ļ�����Խǿ����-Cu����ʱ�����ӴӼס�Cu�����ԼĽ����Դ���ͭ�� ��-Cu����ʱ�����Ӵ�Cu���ң������ҵĽ�����С��ͭ����-Cu����ʱ�����Ӵӱ���Cu�����Ա��Ľ����Դ���ͭ����-Cu����ʱ�����ӴӶ���Cu�����Զ��Ľ����Դ���ͭ���ɴ˷������
��� �⣺A���ס��������Ľ����Ծ�ǿ��Cu�����ǵ�ѹԽ������Խǿ�����Ի������ǣ������ף�����ֻ���ҵĻ���������ͭ�����������ֽ����б��Ļ�ԭ����ǿ����A��ȷ��
B�������ҵĽ�����С��ͭ�����Խ����Ҳ��ܴ�����ͭ��Һ���û���ͭ����B����
C���ס������γ�ԭ���ʱ�����ã����Լ�Ϊ��������C����
D���Ľ����Դ���ͭ���ҵĽ�����С��ͭ�����Խ������Ǽ״����ң��ס����γɺϽ�ʱ�����úϽ�¶���ڿ����У����ȱ���ʴ����D����
��ѡA��
���� ���⿼�鳣�������Ļ��˳��ıȽϷ����Լ������ĵ绯ѧ��ʴ����������Ŀ�Ѷ��еȣ�ע�����ԭ��صĹ���ԭ����ע���ǿ�����ݷ���������������

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
�����Ŀ
7��A��B��C��D��E����Ԫ�ط��������������ڣ���ԭ��������������A��Cͬ���壬���γ����ӻ�����CA��B��Dͬ���壬��D��ԭ��������B��2����������˵����ȷ���ǣ�������
| A�� | BԪ�������ڱ��е�λ��Ϊ�ڶ����ڢ�A�� | |
| B�� | D���������E�ĵ��ʵ�ˮ��Һ����Ư���ԣ���Ư��ԭ����ͬ | |
| C�� | ��A��B��C��D��ɵ������ε���Һ��Ӧ�ɲ���DB2���� | |
| D�� | ����Ԫ�صķǽ�����̬�⻯�ﻹԭ����ǿ����E |
12�����������к�̼����������ߵ��ǣ�������
| A�� | �� | B�� | ��ϩ | C�� | ���� | D�� | ʮ���� |
5������ѧ��Ӧԭ����һ���У�����ѧϰ��������Ҫ�Ķ���ʵ�飮��ش��������⣺
��ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������5mol?L-1 ���ᡢ0.55mol?L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ����Ͳ���¶ȼƣ�
��2��ʵ�����ܷ��û���ͭ˿��������滷�β��������������ܡ�������ԭ���ǽ������ȣ�����ɢʧ��������
��3�����Ǽ�¼��ʵ���������£�
��֪��Q���ţ�=C m��t2-t1������Ӧ����Һ�ı�����CΪ4.18KJ?��-1?Kg-1��mָ��Һ�������������ʵ��ܶȾ�Ϊ1g?cm-3��
�ټ��������H=-56.8KJ/mol�����������3λ��Ч���֣�
�ڸ���ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-56.8KJ/mol��
��Ϊ�˲ⶨ����H2C2O4•2H2O��KHC2O4��K2SO4�������и����ʵ�������������������ʵ�飺
�ٳ�ȡ6.0g��������ˮ�ܽ⣬���250mL������Һ��
������ʽ�ζ�����ȡ25.00mL������Һ������ƿ�У�������2��3�η�̪��Һ����0.2500mol/L NaOH��Һ�ζ�������NaOH��Һ20.00mL��
����ȡ25.00mL������Һ������һ��ƿ�У���0.1000mol/L�����Ը��������Һ�ζ������ĸ��������Һ16.00mL��
�ش��������⣺
��1����֪��0.10mol/L KHC2O4��ҺpHԼΪ3�����к�̼Ԫ�ص�����Ũ���ɴ�С��˳��Ϊc��HC2O4-����c��C2O42-����c��H2C2O4����
��2����ɲ���ƽ�������ӷ���ʽ
5C2O42-+2MnO4-+16H+=10CO2+2Mn2++8H2O��
��3�����������ȡ������Һʱ����ʽ�ζ���������ˮϴ����û����ϴ�����õ�H2C2O4•2H2O����������ƫС�����ƫ����ƫС������Ӱ�족��
��4����������жϵζ��յ�ķ����ǵ������һ����Һ���Ϻ�ɫ�Ұ������ɫ���䣮
��5��������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH�����ʾ��
��i��д��H2C2O4�ĵ��뷽��ʽH2C2O4?H++HC2O4-��HC2O4-?H++C2O42-��
��ii��KHC2O4��Һ�����Ե�ԭ���� �����÷���ʽ����ϱ�Ҫ�����ֻش��С�⣩HC2O4-?H++C2O42-��HC2O4-+H2O?H2C2O4+OH-���������̶ȴ���ˮ��̣�
��iii����0.1mol/L�IJ��������Һ��μ�NaOH��Һ�����ԣ���ʱ��Һ�������Ũ�ȹ�ϵ��ȷ����ad��
a��c��K+���Tc��HC2O4-��+c��H2C2O4��+c��C2O42- �� b��c��Na+���Tc��H2C2O4��+c��C2O42-��
c��c��K+��+c��Na+���Tc��HC2O4-��+c��C2O42- �� d��c��K+����c��Na+��
��ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������5mol?L-1 ���ᡢ0.55mol?L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ����Ͳ���¶ȼƣ�
��2��ʵ�����ܷ��û���ͭ˿��������滷�β��������������ܡ�������ԭ���ǽ������ȣ�����ɢʧ��������
��3�����Ǽ�¼��ʵ���������£�
| ʵ �� �Լ����£� | �� Һ �� �� | �к��ȡ�H | ||||
| t1 | t2 | |||||
| �� | 50mL0.55mol��L-1NaOH | 50mL.0.5mol��L-1HCl | 20�� | 23.3�� | ||
| �� | 50mL0.55mol��L-1NaOH | 50mL.0.5mol��L-1HCl | 20�� | 23.5�� | ||
�ټ��������H=-56.8KJ/mol�����������3λ��Ч���֣�
�ڸ���ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-56.8KJ/mol��
��Ϊ�˲ⶨ����H2C2O4•2H2O��KHC2O4��K2SO4�������и����ʵ�������������������ʵ�飺
�ٳ�ȡ6.0g��������ˮ�ܽ⣬���250mL������Һ��
������ʽ�ζ�����ȡ25.00mL������Һ������ƿ�У�������2��3�η�̪��Һ����0.2500mol/L NaOH��Һ�ζ�������NaOH��Һ20.00mL��
����ȡ25.00mL������Һ������һ��ƿ�У���0.1000mol/L�����Ը��������Һ�ζ������ĸ��������Һ16.00mL��
�ش��������⣺
��1����֪��0.10mol/L KHC2O4��ҺpHԼΪ3�����к�̼Ԫ�ص�����Ũ���ɴ�С��˳��Ϊc��HC2O4-����c��C2O42-����c��H2C2O4����
��2����ɲ���ƽ�������ӷ���ʽ
5C2O42-+2MnO4-+16H+=10CO2+2Mn2++8H2O��
��3�����������ȡ������Һʱ����ʽ�ζ���������ˮϴ����û����ϴ�����õ�H2C2O4•2H2O����������ƫС�����ƫ����ƫС������Ӱ�족��
��4����������жϵζ��յ�ķ����ǵ������һ����Һ���Ϻ�ɫ�Ұ������ɫ���䣮
��5��������0.01mol/L��H2C2O4��KHC2O4��K2C2O4��Һ��pH�����ʾ��
| H2C2O4 | KHC2O4 | K2C2O4 | |
| pH | 2.1 | 3.1 | 8.1 |
��ii��KHC2O4��Һ�����Ե�ԭ���� �����÷���ʽ����ϱ�Ҫ�����ֻش��С�⣩HC2O4-?H++C2O42-��HC2O4-+H2O?H2C2O4+OH-���������̶ȴ���ˮ��̣�
��iii����0.1mol/L�IJ��������Һ��μ�NaOH��Һ�����ԣ���ʱ��Һ�������Ũ�ȹ�ϵ��ȷ����ad��
a��c��K+���Tc��HC2O4-��+c��H2C2O4��+c��C2O42- �� b��c��Na+���Tc��H2C2O4��+c��C2O42-��
c��c��K+��+c��Na+���Tc��HC2O4-��+c��C2O42- �� d��c��K+����c��Na+��
12��ij���Ȼ�ϵİ�ɫ��ĩ���ܺ�������7�ֳ��������е�ij4�֣�NaCl��BaCl2��Mg��NO3��2��K2CO3��CuSO4��NaHCO3��X��������и��ɷֵ����ʵ�����ȣ�������������������Ŀ֮��Ϊ3��2��ijͬѧ��������ʵ�飬��������û���������������ṩ��ʵ���������жԸð�ɫ��ĩ���ж���ȷ���ǣ�������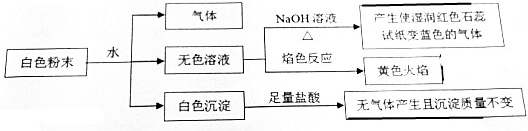

| A�� | һ������BaCl2��Mg��NO3��2��NaHCO3��X����X��NH4HSO4 | |
| B�� | һ������K2CO3��CuSO4��X��NH4Al��SO4��2 | |
| C�� | һ������BaCl2��NaHCO3��X��X��NH4AlO2����һ��ΪNaCl��Mg��NO3��2�е���һ�� | |
| D�� | һ������NaCl��BaCl2��NaHCO3��X����X����ȷ����ʲô���� |

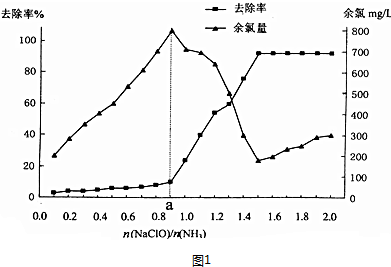
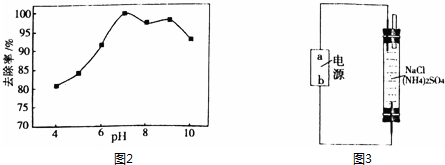


 ��R��ʾ������R���R���ʾ�������⣩
��R��ʾ������R���R���ʾ�������⣩ ��
��
 ��
�� ����дһ�֣�
����дһ�֣�
 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�