��Ŀ����
��֪�����л���ѧ��Ӧ��Ӧ������ͬ�������ɲ�ͬ���л���Ʒ�����磺
�ڱ���ͬϵ����±�ص��ʻ�ϣ����ڹ��������£����������ϵ���ԭ�ӱ�±��ԭ��ȡ�������ڴ��������£�����ijЩλ���ϵ���ԭ�ӱ�±��ԭ��ȡ����
��ҵ������������Ϣ��������·�ߺϳɽṹ��ʽΪ�����ʣ���������һ�����ϡ�


���������·�ߣ��ش��������⣺
(1)A�Ľṹ��ʽ����Ϊ_____________��____________________��
(2)��Ӧ�۵ķ�Ӧ����Ϊ_________________����Ӧ�ݵ�������_______________
(3)��Ӧ�ܵĻ�ѧ����ʽΪ(�л���д�ṹ��ʽ����ע����Ӧ��������ͬ)��
__________________________________________________��
(4)��ҵ�����У��м����A�뾭��Ӧ�ۢܢݵ�D��������ȡ��Aֱ��ת��ΪD�ķ�������ԭ����____________________________��
(5)�����Ʒ�й����ŵĻ�ѧ��Ӧ����ʽΪ��_____________________________��
(6)�������Ͼ��ж���ͬ���칹�壬����ijЩ��������������������ˮ��Һ��FeCl3��Һ����ɫ���� �����ϵ�һ����������֣��۷�����û�м���д��һ�ַ����������������ʿ��ܵĽṹ��ʽ�� ��
��1�� ��2����ȥ��Ӧ ��������ˮ��Һ����
��2����ȥ��Ӧ ��������ˮ��Һ����
��3�� +HBr
+HBr
 ��
��
��4�� ��ˮ�����
��ˮ����� ���ܾ�������Ӧ�õ���Ʒ��
���ܾ�������Ӧ�õ���Ʒ��
��5��



��6��

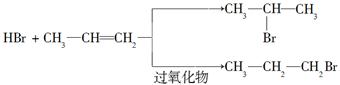
�������������������Ϣ�ں��л��ϳ�����ͼ֪���ڹ��������� ������������ȡ����Ӧ���������ϵ���ԭ�ӱ�±��ԭ��ȡ������
������������ȡ����Ӧ���������ϵ���ԭ�ӱ�±��ԭ��ȡ������ ��B�ܺ�HBr�����ӳɷ�Ӧ����AӦ������ȥ��Ӧ��BΪ
��B�ܺ�HBr�����ӳɷ�Ӧ����AӦ������ȥ��Ӧ��BΪ �����ݲ����֪DΪ
�����ݲ����֪DΪ ����CΪ��
����C��
��1�������Ϸ�����֪AΪ�� ��
��
��2����Ӧ��ΪA������ȥ��Ӧ���� ����
���� Ϊ����ȡ����Ӧ����
Ϊ����ȡ����Ӧ����  ������Ϊ����������ˮ��Һ�����ȣ���Ϊ����ȥ��Ӧ����������ˮ��Һ�����ȣ�
������Ϊ����������ˮ��Һ�����ȣ���Ϊ����ȥ��Ӧ����������ˮ��Һ�����ȣ�
��3���챽��ϩ���廯��ӳ����� ���÷�Ӧ�ķ���ʽΪ��
���÷�Ӧ�ķ���ʽΪ�� +HBr
+HBr
 ��
��
��4�� ��ˮ�����
��ˮ����� ���ܾ�������Ӧ�õ���Ʒ��
���ܾ�������Ӧ�õ���Ʒ��
��5����Ʒ�еĹ�����Ϊȩ��������������Һ������������ͭ����Һ���飻
��6���ɱ����ϵ�һ�ȴ��������֣���FeCl3��Һ����ɫ�����з��ǻ��Ͷ�λ�ı�ϩ���������������ͬ���칹��Ϊ
 ��
��
���㣺�����л���ĺϳɺ��ƶϡ������л������������ʼ��ת����ϵ��

���ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ
�Իش��������⣺
��1��������I��CH3CHO���������л���Ӧ������
��2��������III��������Һ�з�����Ӧ��ѧ����ʽ
��3������˵����ȷ���ǣ�˫ѡ��
| A��������I���Ȼ�����Һ����ɫ |
| B��������II����NaHCO3��Һ��Ӧ |
| C��1mol������IV��ȫȼ������9.5molO2 |
| D��1mol������III����3 mol H2��Ӧ |
���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ��
��д��������X�Ľṹ��ʽ ��д�����֣�
��5���л���R����ͼ��������ӦҲ���Ƶû�����IV����R��NaOH����Һ�з�Ӧ�Ļ�ѧ����ʽΪ ��

���ú˴Ź������ɲⶨ�л�����ӵ���ά�ṹ�����л�������У���ͬλ�õ���ԭ�ӵĺ˴Ź������и�����������Ҳ��ͬ���������������ȷ���л����������ԭ�ӵ��������Ŀ�����磬���ѵĽṹ��ʽΪ��CH3CH2OCH2CH3����˴Ź��������и���������������������ͼ��ʾ��
CH3CH2OCH2CH3�ĺ˴Ź�������
��1�����������У���˴Ź�����������������ֻ��һ������________��
| A��CH3CH3 | B��CH3COOH | C��CH3COOCH3 | D��CH3COCH3 |

A�ĺ˴Ź�������
��3���ú˴Ź������ķ������о�C2H6O�Ľṹ�����Ҫ˵�����ݺ˴Ź������Ľ����ȷ��C2H6O���ӵĽṹ��ʽ�ķ�����___________________________________________________________��
���л�������У���ͬλ�õ���ԭ�ӵĺ˴Ź������и�����������ֵ(�ź�)Ҳ��ͬ�����ݷ�ֵ(�ź�)����ȷ���л����������ԭ�ӵ��������Ŀ��
(1)�����л�������У���˴Ź�������ͼ��ֻ��1�����������________��
| A��CH3��CH3 | B��CH3COOH | C��CH3COOCH3 | D��CH3COCH3 |


 �ĵ��壬��A�����������ŵ�������______ ��
�ĵ��壬��A�����������ŵ�������______ �� ��_______��_______��_______��
��_______��_______��_______��
 R1-CHO+R2-CHO
R1-CHO+R2-CHO


