��Ŀ����
7��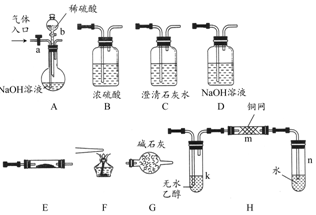 ��ͼ��ʾΪ���������Ʊ������롢�����������֤�IJ�������װ�ã������豸���г̶ֹ�װ�þ���ȥ���������Ҫ��������и��⣨����װ�ÿ�����ѡ�ã���Ҫʱ���ظ�ѡ��
��ͼ��ʾΪ���������Ʊ������롢�����������֤�IJ�������װ�ã������豸���г̶ֹ�װ�þ���ȥ���������Ҫ��������и��⣨����װ�ÿ�����ѡ�ã���Ҫʱ���ظ�ѡ����1�����������ͨ��CO��CO2�Ļ�����壬E�ڷ���CuO��ѡ��װ�û�ô��������CO������֤�仹ԭ�Լ����������ѡװ�õ�����˳��ΪACBECF������ĸ��������֤CO���������������AB֮���Cװ������Һ���ֳ��壬EF֮���Cװ������Һ����ǣ�
��2��ֹͣCO��CO2��������ͨ�룬E�ڷ���Na2O2����A-��E-��D-��B-��Hװ��˳����ȡ���������O2������O2�����Ҵ�����ʱ������aӦ�رգ�����bӦ����Ҫ���ȵ�����װ����k��m������ţ���m�з�Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ��1����NaOH��Һ����ʯ��ˮ��ȥCO2����Ũ�������CO����CuO����CO���ó���ʯ��ˮ����CO���������ȼ�շ���ȥ�����CO��
��2��ֹͣCO��CO2��������ͨ�룬aӦ�رգ�b��Ũ������NaOH��Һ��Ӧ�ų��������ӿ�A�е�ˮ��������H2O��E��Na2O2��Ӧ������O2������k������CH3CH2OH�Ļӷ�������m��CH3CH2OH��O2��Ӧ����CH3CHO��
��� �⣺��1��Ҫ��ô��������CO�ͱ�����A�е�NaOH��Һ����CO2����ͨ��C�еij���ʯ��ˮ�����֤��CO2�ѱ���ȫ���գ���ͨ��BŨ�������CO���壮COͨ��E�м��ȵ�CuO��������CO2����C�г���ʯ��ˮ���ձ���ǣ�֤��CO��ԭ�Լ����������ѡװ�õ�����˳��ΪACBECF��
�ʴ�Ϊ��ACBECF��AB֮���Cװ������Һ���ֳ��壬EF֮���Cװ������Һ����ǣ�
��2��ֹͣCO��CO2��������ͨ���Ҫ�رջ���a������b����ϡH2SO4��NaOH �����кͷ�Ӧ��������ˮ������Aװ���г�������E��Na2O2��Ӧ�ͻ���O2���ɣ�������NaOH����D�У�O2��B��ŨH2SO4�����ٽ���Hװ�ý��Ҵ�������O2���ؼ��ȵ�ͭ˿������������ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ���رգ���k��m��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ���⿼����������Ʊ������롢�����������֤���Լ��Ҵ�������������Ӧ�ȣ���Ŀ�Ѷ��еȣ�ע�����������Ʊ�������ʵ��ԭ���������ڿ���ѧ���ķ���������ʵ��̽��������

| ѡ�� | �ı������ | ���� |
| A | ���� | CO32-��ˮ��ƽ�������ƶ� |
| B | ����AlCl3���� | ������������ |
| C | ����100mLH2O | ��Һ��c��H+����c��OH-������С |
| D | ��������CH3COONa���� | ��Һ��n��CO32-������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Na0H�����ڿ����б��� | B�� | �������� | ||
| C�� | ���ʵ�ȼ�� | D�� | ֲ��Ĺ������ |
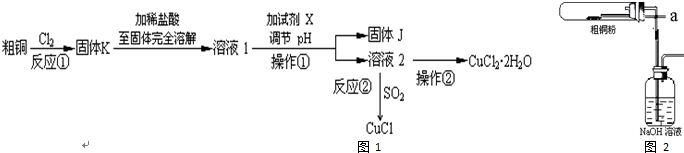


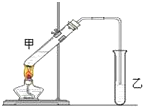 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ洢����������������ζ��������������ʵ����������Ҳ��������ͼ��ʾװ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ洢����������������ζ��������������ʵ����������Ҳ��������ͼ��ʾװ����ģ��ù��̣���ش��������⣺