��Ŀ����
 ��ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺
��ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺I��һ���¶��£���1L�ܱ������м���1mol HI��g����������Ӧ2HI?H2+I2��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0��2min�ڵ�ƽ����Ӧ����v��HI��=
���¶��£�H2��g��+I2��g��?2HI��g����ƽ�ⳣ��K=
a��ƽ�ⳣ�� b��HI��ƽ��Ũ�� c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
�����£���pH=3������aL�ֱ�������������Һ��ϣ������Һ�������ԣ�
��Ũ��Ϊ1.0��10-3mol?L-1�İ�ˮbL��
��c��OH-��=1.0��10-3mol?L-1�İ�ˮcL��
��c��OH-��=1.0��10-3mol?L-1������������ҺdL��
��a��b��c��d֮���ɴ�С�Ĺ�ϵ��
��ҵ��ˮ�г�����һ������CrO42-�����ǻ�����弰��̬ϵͳ�����ܴ���˺���������д�������ԭ�������dz��õ�һ�ַ�����
CrO
2- 4 |
| H+ |
| ת�� |
2- 7 |
| Fe2+ |
| ��ԭ |
| CH- |
| ���� |
��1��ת�������д���ƽ��2CrO42-+2H+? Cr2O72-+H2O����˵����Ӧ�ﵽƽ��״̬����
��ţ���
A��CrO42-��Cr2O72-��Ũ����ͬ
B.2v��Cr2O72-��=v��CrO42-��
C����Һ��pH���ֲ���
D����Һ��ɫ���ֲ���
��2����1Lת����������Һ�к���Ԫ������Ϊ28.6g��CrO42-?��10/llת��ΪCr2O72-��
��ת����������Һ�У�c��Cr2O72-��=
����֪�������£��÷�Ӧ��ƽ�ⳣ��K=1014������ת����������Һ��pHΪ
��3����������Ksp[Cr��OH��3]=10-32��Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����
���㣺��ѧƽ��ļ���,��ѧƽ���Ӱ������,��ѧƽ��״̬���ж�
ר�⣺
�����������ݻ�ѧ��Ӧ���ʼ����һ���գ�����ƽ�ⳣ�����㷽�������ڶ����գ�����ƽ���ƶ�ԭ�������������գ�
��pH=3����������ʵ���Ũ��=1��10-3 mol/L���к���ͬ���ʵ��������ᣬ���Ũ��Խ�����õļ�Խ�٣�ע��������Һ�����������ӵ����ʵ���Ũ�Ⱥͼ��Ũ�Ȳ��ȣ�
��1�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣬
A��Cr2O72-��CrO42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣻
B��2v��Cr2O72-��=v��CrO42-���������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣻
C����Һ��pHֵ���ֲ��䣬˵��������Ũ�Ȳ��䣬���ж�ƽ�⣻
D����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣻
��2���ữʱ�����ķ�ӦΪ��2CrO42-+2H+?Cr2O72-+H2O��-
�����غ��й�ϵʽ��2Cr��2CrO42-��Cr2O72-����ʽ�����n��Cr2O72-����n��CrO42-��ʣ����
��������ữ��������Һ��c��Cr2O72-��
�ڸ��ݷ�Ӧ 2CrO42-+2H+�T?Cr2O72-+H2O
��ƽ�ⳣ��K�T1014�������c��H+��=1.0��10-6mol����PH=6��
��3�������ܶȻ�������ʽ���㣮
��pH=3����������ʵ���Ũ��=1��10-3 mol/L���к���ͬ���ʵ��������ᣬ���Ũ��Խ�����õļ�Խ�٣�ע��������Һ�����������ӵ����ʵ���Ũ�Ⱥͼ��Ũ�Ȳ��ȣ�
��1�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣬
A��Cr2O72-��CrO42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣻
B��2v��Cr2O72-��=v��CrO42-���������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣻
C����Һ��pHֵ���ֲ��䣬˵��������Ũ�Ȳ��䣬���ж�ƽ�⣻
D����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣻
��2���ữʱ�����ķ�ӦΪ��2CrO42-+2H+?Cr2O72-+H2O��-
�����غ��й�ϵʽ��2Cr��2CrO42-��Cr2O72-����ʽ�����n��Cr2O72-����n��CrO42-��ʣ����
��������ữ��������Һ��c��Cr2O72-��
�ڸ��ݷ�Ӧ 2CrO42-+2H+�T?Cr2O72-+H2O
��ƽ�ⳣ��K�T1014�������c��H+��=1.0��10-6mol����PH=6��
��3�������ܶȻ�������ʽ���㣮
���
�⣺����ͼ���֪��0-2 min�ڣ����������ʵ���������0.1mol�����Ȼ����HI��0.2mol��ʹ��ƽ����Ӧ����v��HI��=
=0.1 mol/��L?min����ƽ��ʱ�����͵��Ũ�ȶ���0.1mol/L�����⻯�����0.8mol/L��ʹ�ø��¶���ƽ�ⳣ��K=
�������淴Ӧ��ƽ�ⳣ����64��ƽ�ⳣ��ֻ���¶��й�ϵ��ѡ��a����ȷ�����ڷ�Ӧǰ��������䣬���Ը÷�Ӧ�ǵ�Ч�ģ����ѡ��b��ȷ��d����ȷ��Ũ�����ӣ���Ӧʱ�����ﵽƽ���ʱ����٣�ѡ��c����ȷ����ѡb��
�ʴ�Ϊ��0.1mol/��L?min���� 64��b��
��pH=3����������ʵ���Ũ��=1��10-3 mol/L��
���а�ˮ�����ʵ���Ũ����1��10-3 mol/L����һˮ�ϰ���������ʣ�ֻ�в��ֵ��룬���Ԣ��а�ˮ��Ũ�ȴ���1��10-3 mol/L���������������ӵ�Ũ����1��10-3 mol/L��
�����������������ǿ����ʣ������Ӻ������������к�ʱ��1��1�Ĺ�ϵ�������Ӻ����������ӵ�Ũ����ȣ�����a��d�������ȣ���a=d��
�ڵİ�ˮŨ�ȴ��ڢٵ�Ũ�ȣ��к���ͬ���ʵ��������ᣬ��ˮ��Ũ��Խ��ʹ�õİ�ˮ�����ԽС������c��b������Ͱ�ˮ��Ӧ���ɵ��Ȼ����ǿ�������Σ�ˮ���ʹ��Һ�����ԣ�Ҫ��ʹ��Һ�����ԣ���ˮ�����ʵ���Ӧ��������Ĵ�Щ��������Ũ�ȺͰ�ˮ��Ũ�����ʱ����ˮ�����bӦ������������a������Һ�����a��b��
�ڢ������������ӵ�Ũ����ȣ�һˮ�ϰ���һԪ������ʣ�����������ǿ����ʣ�����ˮ��Ũ�ȴ��ڢ�������������Ũ�ȣ��к���ͬ���ʵ�����������ʱ�������õİ�ˮ�����С�ڢ�����������Һ���������c��d=a��
����a��b��c��d�Ĺ�ϵb��a=d��c��
�ʴ�Ϊ��b��a=d��c��
��1�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣬
A��Cr2O72-��CrO42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣬��A����
B��2v��Cr2O72-��=v��CrO42-���������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��B����
C����Һ��pHֵ���ֲ��䣬˵��������Ũ�Ȳ��䣬���ж�ƽ�⣬��C��ȷ��
D����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��D��ȷ��
�ʴ�Ϊ��CD��
��2���ữʱ�����ķ�ӦΪ��2CrO42-+2H+?Cr2O72-+H2O����1L�ữ��������Һ�к���Ԫ�ص�����Ϊ28.6g��CrO42-��
ת��ΪCr2O72-��˵����Ԫ��
ת��ΪCr2O72-
�����غ��й�ϵʽ��2Cr��2CrO42-��Cr2O72-
2 1
n��Cr2O72-��
��n��Cr2O72-��=0.25mol��n��CrO42-��ʣ��=0.05mol��
����ữ��������Һ��c��Cr2O72-��=
=0.25mol?L-1�� c��CrO42-��ʣ��=0.05mol?L-1
����H+�����ʵ���Ũ��Ϊamol/L��
2CrO42-+2H+�T?Cr2O72-+H2O
ƽ�⣨mol/L�� 0.05 a 0.25
ƽ�ⳣ��K=
�T1014��
��a=1.0��10-6mol��PH=6��
�ʴ�Ϊ��0.25mol/L��6��
��3��������Ksp[Cr��OH��3]=1��10-32��Ҫʹ�������ˮ��c��Cr3+������1��10-5mol/L����c��Cr3+����c3��OH-��=1��10-32��c��OH-��=1��10-9mol/L��pH=5��
�ʴ�Ϊ��5��
| 0.2mol |
| 1L��2min |
| 0.1mol/L��0.1mol/L |
| (0.8mol/L)2 |
| 1 |
| 64 |
�ʴ�Ϊ��0.1mol/��L?min���� 64��b��
��pH=3����������ʵ���Ũ��=1��10-3 mol/L��
���а�ˮ�����ʵ���Ũ����1��10-3 mol/L����һˮ�ϰ���������ʣ�ֻ�в��ֵ��룬���Ԣ��а�ˮ��Ũ�ȴ���1��10-3 mol/L���������������ӵ�Ũ����1��10-3 mol/L��
�����������������ǿ����ʣ������Ӻ������������к�ʱ��1��1�Ĺ�ϵ�������Ӻ����������ӵ�Ũ����ȣ�����a��d�������ȣ���a=d��
�ڵİ�ˮŨ�ȴ��ڢٵ�Ũ�ȣ��к���ͬ���ʵ��������ᣬ��ˮ��Ũ��Խ��ʹ�õİ�ˮ�����ԽС������c��b������Ͱ�ˮ��Ӧ���ɵ��Ȼ����ǿ�������Σ�ˮ���ʹ��Һ�����ԣ�Ҫ��ʹ��Һ�����ԣ���ˮ�����ʵ���Ӧ��������Ĵ�Щ��������Ũ�ȺͰ�ˮ��Ũ�����ʱ����ˮ�����bӦ������������a������Һ�����a��b��
�ڢ������������ӵ�Ũ����ȣ�һˮ�ϰ���һԪ������ʣ�����������ǿ����ʣ�����ˮ��Ũ�ȴ��ڢ�������������Ũ�ȣ��к���ͬ���ʵ�����������ʱ�������õİ�ˮ�����С�ڢ�����������Һ���������c��d=a��
����a��b��c��d�Ĺ�ϵb��a=d��c��
�ʴ�Ϊ��b��a=d��c��
��1�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣬
A��Cr2O72-��CrO42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣬��A����
B��2v��Cr2O72-��=v��CrO42-���������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��B����
C����Һ��pHֵ���ֲ��䣬˵��������Ũ�Ȳ��䣬���ж�ƽ�⣬��C��ȷ��
D����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��D��ȷ��
�ʴ�Ϊ��CD��
��2���ữʱ�����ķ�ӦΪ��2CrO42-+2H+?Cr2O72-+H2O����1L�ữ��������Һ�к���Ԫ�ص�����Ϊ28.6g��CrO42-��
| 10 |
| 11 |
| 10 |
| 11 |
�����غ��й�ϵʽ��2Cr��2CrO42-��Cr2O72-
2 1
28.6��
| ||
| 52 |
��n��Cr2O72-��=0.25mol��n��CrO42-��ʣ��=0.05mol��
����ữ��������Һ��c��Cr2O72-��=
| n |
| V |
����H+�����ʵ���Ũ��Ϊamol/L��
2CrO42-+2H+�T?Cr2O72-+H2O
ƽ�⣨mol/L�� 0.05 a 0.25
ƽ�ⳣ��K=
| 0.25 |
| 0.052��a2 |
��a=1.0��10-6mol��PH=6��
�ʴ�Ϊ��0.25mol/L��6��
��3��������Ksp[Cr��OH��3]=1��10-32��Ҫʹ�������ˮ��c��Cr3+������1��10-5mol/L����c��Cr3+����c3��OH-��=1��10-32��c��OH-��=1��10-9mol/L��pH=5��
�ʴ�Ϊ��5��
������������Ҫ�����˻�ѧ��Ӧ���ʵļ��㡢��ѧ��Ӧƽ�ⳣ���ļ��㡢�����ʱ�Ķ����жϣ��Ѷ��еȣ�ע�����һԪ����Һ�����������ӵ����ʵ���Ũ�Ⱥͼ��Ũ�Ȳ��ȣ�

��ϰ��ϵ�д�
�����Ŀ
�ɶ�����Ԫ�صļ������γɵ�ij�����һ�������Ӻ�һ�������Ӻ��������֮��Ϊ20������˵������ȷ���ǣ�������
| A�������������Ӻ������Ӹ�����һ����� |
| B��������һ�������Ӽ��������й��ۼ� |
| C������Ԫ��һ������ͬһ���ڣ�Ҳ����ͬһ���� |
| D�������������Ӱ뾶һ�����������Ӱ뾶 |
��ѧ�ҽ��������Ƴ�һ������ϸ��ȼ�ϵ�أ�����ϸ�����л���ת��Ϊ���飬Ȼ����ͨ����KOHΪ����ʵ�ȼ�ϵ�ط��磮��ظ�����ӦΪ��������
| A��CH4-8e-+8OH-=CO2+6H2O |
| B��O2+4H++4e-=2H2O |
| C��CH4+10OH--8e-=C32-+7H2O |
| D��O2+2H2O+4e-=4OH- |
����Ԫ�����ʵĵݱ������ȷ���ǣ�������
| A����һ�����ܣ�B��Be��Mg��Na |
| B��Ԫ�صĵ縺�ԣ�O��N��S��P |
| C����̬�⻯����ȶ��ԣ�NH3��CH4��PH3��SiH4 |
| D��ԭ�Ӱ뾶��Be��B��C��N |
������������Ҫ�õ��¶ȼƵ��ǣ�������
| A������NaCl��SiO2 |
| B��.����Һ�������õ����� |
| C��.����ƾ���ˮ |
| D��.�������ͺ�ˮ |
���¹���ʵ�������˵����ȷ���ǣ�������
| A������ʱΪ�ӿ��ٶȿ����ò����������� |
| B������ʱ���������ʯ������ |
| C�����þƾ���ȡ��ˮ�еĵ� |
| D������ʱ�¶ȼ�Һ��Ӧ��������ƿ֧�ܿ�ƽ�� |
�������ƺͶ��ȷӣ� ���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
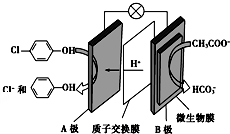
 ���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ��������
| A��B�������� |
| B��B�������� |
| C��ÿת��2mol���ӣ���1molCH3COO-������ |
D��A���缫��ӦʽΪ��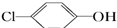 +2e-+H+ +2e-+H+ +Cl- +Cl- |
ѡ��������Ũ����ķ�Ӧ������ߵ�ϩ����������
A�� |
B�� |
| C���T |
