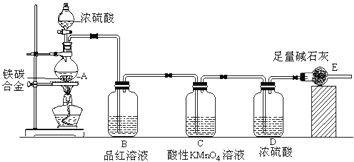
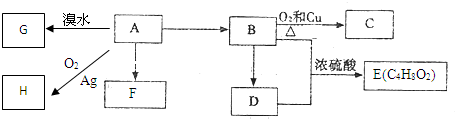
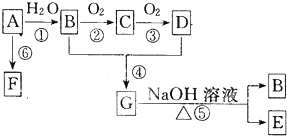
��Ŀ����
��ҵ���Ƶõĺ��������п��ܺ����������������ƺ����������ʣ�Ϊ�˲ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ�������Һ30mL����ַ�Ӧ����˳�������Һʹ���ɵ�SO2ȫ���ݳ���Na2S2O3+H2SO4��Na2SO4+SO2��+S��+H2O��������й�ʵ���������£���״������
��1����������������Һ�����ʵ���Ũ�ȣ���д��������̣�
��2����������ʵ�����ݣ����жϸ���Ʒ ������ĸ����
a������Na2SO3��Na2SO4b������Na2SO3��Na2SO4
c����Na2SO3����Na2SO4 d����Na2SO3��Na2SO4
��3������30.16g����Ʒ��һ����������������Һ����ȣ������ۣ�����������������a L���ڲ�ͬȡֵ��Χʱ������SO2�����b L����̬����ֵ�������ú�a��b�Ĵ���ʽ��ʾ��
| ��һ�� | �ڶ��� | ������ | |
| ��Ʒ������/g | 7.54 | 15.08 | 35.00 |
| ������������/L | 0.672 | 1.344 | 2.688 |
| �������/g | 0.80 | 1.60 | 3.20 |
��2����������ʵ�����ݣ����жϸ���Ʒ
a������Na2SO3��Na2SO4b������Na2SO3��Na2SO4
c����Na2SO3����Na2SO4 d����Na2SO3��Na2SO4
��3������30.16g����Ʒ��һ����������������Һ����ȣ������ۣ�����������������a L���ڲ�ͬȡֵ��Χʱ������SO2�����b L����̬����ֵ�������ú�a��b�Ĵ���ʽ��ʾ��
���㣺̽�����ʵ���ɻ�������ʵĺ���,�������ʵ����ʼ��ۺ�Ӧ��
ר�⣺ʵ��̽�������ݴ�����
��������1��ͨ��ͼ�����ݷ���������ʵ�����ݱ�����������ȫ��Ӧ���йػ�ѧ����ʽΪ��Na2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O���ɼ��������ᷢ���Ǹ���Ӧ���й�ϵʽH2SO4��SO2��n��SO2��=n��H2SO4��=
=0.12mol��c=
��
��2��n��s��=0.1mol������ݻ�ѧ����ʽNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O����֪n��Na2S2O3��=0.1mol��m��Na2S2O3 ?5H2O��=24.8g�����ɶ��������������ʵ���Ϊ0.1mol����Na2SO3���ɵĶ����������ʵ���=0.12mol-0.1mol=0.02mol��n��Na2SO3��=0.02mol��m��Na2SO3��=0.02mol��126g/mol=2.52g������m��Na2S2O3 ?5H2O��+m��Na2SO3��=24.8g+2.52g=27.32g��35.00g�����Թ����к���Na2SO4��
��3��ͨ��ͼ�����ݷ���������ʵ�����ݿ�֪��30ml������ǡ������Ʒ��ȫ��Ӧʱ��������Ϊ30.16g�����0��a��0.03ʱ������������ų������������������㣬b=��2.688L+0.03����a=89.6a����a��0.03�����������Ʒ��ȫ��Ӧ�������������b=2.688��
| 2.688L |
| 22.4L/mol |
| n |
| V |
��2��n��s��=0.1mol������ݻ�ѧ����ʽNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O����֪n��Na2S2O3��=0.1mol��m��Na2S2O3 ?5H2O��=24.8g�����ɶ��������������ʵ���Ϊ0.1mol����Na2SO3���ɵĶ����������ʵ���=0.12mol-0.1mol=0.02mol��n��Na2SO3��=0.02mol��m��Na2SO3��=0.02mol��126g/mol=2.52g������m��Na2S2O3 ?5H2O��+m��Na2SO3��=24.8g+2.52g=27.32g��35.00g�����Թ����к���Na2SO4��
��3��ͨ��ͼ�����ݷ���������ʵ�����ݿ�֪��30ml������ǡ������Ʒ��ȫ��Ӧʱ��������Ϊ30.16g�����0��a��0.03ʱ������������ų������������������㣬b=��2.688L+0.03����a=89.6a����a��0.03�����������Ʒ��ȫ��Ӧ�������������b=2.688��
���
�⣺��1��ͨ��ͼ�����ݷ���������ʵ�����ݱ�����������ȫ��Ӧ���йػ�ѧ����ʽΪ��Na2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O���ɼ��������ᷢ���Ǹ���Ӧ���й�ϵʽH2SO4��SO2��n��SO2��=n��H2SO4��=
=0.12mol��c=
=
=4mol/L��
������������Һ�����ʵ���Ũ��4mol/L��
��2��n��s��=0.1mol������ݻ�ѧ����ʽNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O����֪n��Na2S2O3��=0.1mol��m��Na2S2O3 ?5H2O��=24.8g�����ɶ��������������ʵ���Ϊ0.1mol����Na2SO3���ɵĶ����������ʵ���=0.12mol-0.1mol=0.02mol��n��Na2SO3��=0.02mol��m��Na2SO3��=0.02mol��126g/mol=2.52g������m��Na2S2O3 ?5H2O��+m��Na2SO3��=24.8g+2.52g=27.32g��35.00g�����Թ����к���Na2SO4��ѡd��
�ʴ�Ϊ��d��
��3��ͨ��ͼ�����ݷ���������ʵ�����ݿ�֪��30ml������ǡ������Ʒ��ȫ��Ӧʱ��������Ϊ30.16g�����0��a��0.03ʱ������������ų������������������㣬b=��2.688L+0.03����a=89.6a����a��0.03�����������Ʒ��ȫ��Ӧ�������������b=2.688��4
�𣺵���������������a L���ڲ�ͬȡֵ��Χʱ������SO2�����b L����̬����ֵ��0��a��0.03ʱb=89.6a��a��0.03ʱb=2.688��
| 2.688L |
| 22.4L/mol |
| n |
| V |
| 0.12mol |
| 0.03L |
������������Һ�����ʵ���Ũ��4mol/L��
��2��n��s��=0.1mol������ݻ�ѧ����ʽNa2S2O3+H2SO4=Na2SO4+S��+SO2��+H2O����֪n��Na2S2O3��=0.1mol��m��Na2S2O3 ?5H2O��=24.8g�����ɶ��������������ʵ���Ϊ0.1mol����Na2SO3���ɵĶ����������ʵ���=0.12mol-0.1mol=0.02mol��n��Na2SO3��=0.02mol��m��Na2SO3��=0.02mol��126g/mol=2.52g������m��Na2S2O3 ?5H2O��+m��Na2SO3��=24.8g+2.52g=27.32g��35.00g�����Թ����к���Na2SO4��ѡd��
�ʴ�Ϊ��d��
��3��ͨ��ͼ�����ݷ���������ʵ�����ݿ�֪��30ml������ǡ������Ʒ��ȫ��Ӧʱ��������Ϊ30.16g�����0��a��0.03ʱ������������ų������������������㣬b=��2.688L+0.03����a=89.6a����a��0.03�����������Ʒ��ȫ��Ӧ�������������b=2.688��4
�𣺵���������������a L���ڲ�ͬȡֵ��Χʱ������SO2�����b L����̬����ֵ��0��a��0.03ʱb=89.6a��a��0.03ʱb=2.688��
���������⿼����������ɵ�̽�����������ݷ����жϣ���Ӧ����ͷ�Ӧ���̵�����Ӧ�ã����������Ϣ�ǽ���ؼ�����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
�����йػ�ѧ��Ӧ�����������ǣ�������
| A�������κ�ˮ�ķ�Ӧһ�����кͷ�Ӧ |
| B�����ֽⷴӦһ��û�е��ʲμ� |
| C������һ�ֵ��ʺ�һ�ֻ�����ķ�Ӧһ�����û���Ӧ |
| D��������֮��һ�����ܷ�����Ӧ |
�����顢�⾧�����ϩ�ǽ����������л��ϳɷ����Ʊ��ľ�������ͼ��ʾ����ṹ�Ļ�״�л���������л����˵����ȷ���ǣ�������

| A�������顢�⾧�����ϩ������ʹ��ˮ��ɫ |
| B���⾧�����ϩ��Ϊͬ���칹�� |
| C��������Ķ��ȴ��ﹲ��3�� |
| D�������顢�⾧����Cԭ�Ӷ��γ�4��������������Ƕ��������� |
 �ⶨNaOH��Һ��Ũ�ȣ���ÿ�ζ���ȷȡ���ڱ����������Ϊ0.2040g���ζ��յ�ʱ��Һ��pHԼΪ9.1��pH�Ʋ��������ζ�ʱ�÷�̪��ָʾ�����ô���NaOH��Һ�ζ��ڱ���������أ�������������ʵ�飬ÿ����������������Һ��������±���
�ⶨNaOH��Һ��Ũ�ȣ���ÿ�ζ���ȷȡ���ڱ����������Ϊ0.2040g���ζ��յ�ʱ��Һ��pHԼΪ9.1��pH�Ʋ��������ζ�ʱ�÷�̪��ָʾ�����ô���NaOH��Һ�ζ��ڱ���������أ�������������ʵ�飬ÿ����������������Һ��������±���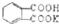 ��ʽ��Ϊ204.0����1mol�ڱ��������������1mol NaOH��Ӧ��
��ʽ��Ϊ204.0����1mol�ڱ��������������1mol NaOH��Ӧ��