��Ŀ����
ͨ��ѧϰ������ʣ���У��ѧ��ȤС���ͬѧ��ʵ���ҵġ����������Ƿ�����Լ���ֵij̶ȡ�չ����̽���������һͬ���룺
��1����������⡿�����������Ƿ���ʣ�
��2������������衿��ͬѧ�Ǿ���������Ϊ��Ʒ�������ֿ��������û�б��ʣ����ֱ��ʣ���ȫ���ʣ�
��3����ʵ��̽������
��4����С����˼������
��С��ͬѧ��������ʵ�鷽������ʵ�飬ȷ������Ʒ�Ѳ��ֱ��ʣ���д��ʵ���з�Ӧ�Ļ�ѧ����ʽ�� ��
�����������׳���ʯ�ң�ũҵ�ϳ����� ��
��5��80��5%������������Һ��100��ϡ����ǡ����ȫ��Ӧ����ϡ���������ʵ�����������
��1����������⡿�����������Ƿ���ʣ�
��2������������衿��ͬѧ�Ǿ���������Ϊ��Ʒ�������ֿ��������û�б��ʣ����ֱ��ʣ���ȫ���ʣ�
��3����ʵ��̽������
| ʵ�鲽�輰���� | ʵ������ | ʵ����� |
| ȡ�����Թ��У�������������ˮ������ ��ȡ�ϲ���Һ��������ɫ��̪��Һ �ڵ�ȥ�ϲ���Һ�������Թ���ע�� ϡ���� |
����ɫ��̪��Һ��� �� |
���ֱ��� |
| ����ɫ��̪��Һ����� �� |
||
| �� ��û�����ݲ��� |
��С��ͬѧ��������ʵ�鷽������ʵ�飬ȷ������Ʒ�Ѳ��ֱ��ʣ���д��ʵ���з�Ӧ�Ļ�ѧ����ʽ��
�����������׳���ʯ�ң�ũҵ�ϳ�����
��5��80��5%������������Һ��100��ϡ����ǡ����ȫ��Ӧ����ϡ���������ʵ�����������
���㣺ҩƷ�Ƿ���ʵ�̽��,�й��������������ļ���,��Ļ�ѧ����,��Ļ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���,��ѧ̽��
��������3������������������ж�����̼��Ӧ����̼��ƣ���������������Һ�Լ��ԣ���ʹ����̪��죬���÷�̪�������Ƿ���ڣ�̼��ƿ������ᷴӦ���ɶ�����̼���ʿ���ͨ�������ᷴӦ�Ƿ��������ж�̼����Ƿ���ڣ������ʵ����ۡ�����������
��4�����������غ㶨��л��ѧ����ʽ�������������Ƶ����ʽ��
��5������ϡ�������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��Ӧ�Ļ�ѧ����ʽ�����������Ƶ�����������������Ȼ��⼰��Ӧ��������Һ�������Ȼ��Ƶ�������������Һ�����������������㹫ʽ�������Һ���������������ļ��㣮
��4�����������غ㶨��л��ѧ����ʽ�������������Ƶ����ʽ��
��5������ϡ�������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��Ӧ�Ļ�ѧ����ʽ�����������Ƶ�����������������Ȼ��⼰��Ӧ��������Һ�������Ȼ��Ƶ�������������Һ�����������������㹫ʽ�������Һ���������������ļ��㣮
����⣺��3�����ݽ��ۣ����ֱ��ʣ���ô�ɷ����������ƺ�̼��ƣ��ʡ��ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᡱ��϶������ݲ������������ȡ�ϲ���Һ��������ɫ��̪��Һ����ɫ��̪��Һ����죬˵���������������ƣ������ݲ�����˵��ȫ��̼��ƣ��ʽ����ǣ���ȫ���ʣ��ɽ��۲���ٳ�������û�����ݲ�����˵����������δ���ʣ���ȫ�����������ƣ��ʢ�ȡ�ϲ���Һ��������ɫ��̪��Һ����ɫ��̪��Һ��죻
��4��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2�������������Լ��ԣ��������п�������������������
��5���⣺��ϡ��������������Ϊx��Ӧ�����������Ƶ�����=80g��5%=4g
NaOH+HCl�TNaCl+H2O
40 36.5
4g x
=
x=3.65g ϡ���������ʵ���������=
��100%=3.65%
��ϡ���������ʵ�����������3.65%
�ʴ�Ϊ��
��3��
��4��CaCO3+2HCl=CaCl2+H2O+CO2����������������
��5��3.65%
��4��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2�������������Լ��ԣ��������п�������������������
��5���⣺��ϡ��������������Ϊx��Ӧ�����������Ƶ�����=80g��5%=4g
NaOH+HCl�TNaCl+H2O
40 36.5
4g x
| 40 |
| 4g |
| 36.5 |
| x |
| 3.65g |
| 100g |
��ϡ���������ʵ�����������3.65%
�ʴ�Ϊ��
��3��
| ʵ�鲽�輰���� | ʵ������ | ʵ����� |
| ȡ�����Թ��У�������������ˮ������ ��ȡ�ϲ���Һ��������ɫ��̪��Һ �ڵ�ȥ�ϲ���Һ�������Թ���ע�� ϡ���� |
����ɫ��̪��Һ��� �������ݲ��� |
���ֱ��� |
| ����ɫ��̪��Һ����� �������ݲ��� |
��ȫ���� | |
| ����ɫ��̪��Һ��� ��û�����ݲ��� |
û�б��� |
��5��3.65%
�����������������Ʊ��ʵ�ԭ����������ơ�̼��Ƶ������ǽ������ǰ��ͻ��������⣬��Ҫ��������������ۣ����߸��ݽ����Ƴ�������ȷ��д��ѧ����ʽ���ݻ�ѧ����ʽ���Ա�ʾ��Ӧ�и����ʵ������ȣ��ɷ�Ӧ��ij���ʵ������ɼ�����������ʵ�������

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
�����Ŀ
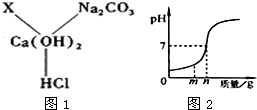 ��1��С��ͬѧ�ڸ�ϰCa��OH��2��ѧ����ʱ�����ɳ���Ca��OH��2���������ʼ����Ӧ�Ĺ�ϵ��ͼ1��ʾ��ͼ�С�--����ʾ���������������ܷ�����Ӧ����
��1��С��ͬѧ�ڸ�ϰCa��OH��2��ѧ����ʱ�����ɳ���Ca��OH��2���������ʼ����Ӧ�Ĺ�ϵ��ͼ1��ʾ��ͼ�С�--����ʾ���������������ܷ�����Ӧ����