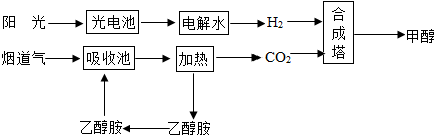
��Ŀ����
6��Ϊ�ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ij��ѧ��ȤС����������ʵ�飬��ƽ���������±�������Ʒ�е����ʲ��μӷ�ӦҲ���ܽ���ˮ����ش��������⣺| ���ʵ����� | ƽ��ֵ |
| ��Ӧǰ���ձ�+���� | 222.0g |
| ʯ��ʯ��Ʒ | 10.0g |
| ��Ӧ���ձ�+��Һ | 226.2g |
��2�������ʯ��ʯ��Ʒ��̼��Ƶ�������������ȷ��0.1%����д����Ҫ�ļ�����̣�
���� ��1��������Ԫ����ɣ�һ��Ԫ������Ԫ�صĻ������������������ָ���������������ȫ���������ӵĻ��������ָ�������������ȫ�������������ӵĻ��������ָ������������Ӻ�������ӵĻ����
��2�����������������Һ�������仯�����⣮
��� �⣺��1��̼��ƶ����ɽ������Ӻ����������ɵĻ�����������Σ������
��2����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2�� ��������
100 44 100-44=56
x 226.2g-222.0g=4.2g
$\frac{100}{x}$=$\frac{56}{4.2g}$
x=7.5g
��ʯ��ʯ��Ʒ��̼��Ƶ���������$\frac{7.5g}{10g}$��100%=75.0%
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ���������75.0%��
���� �����ǶԻ�ѧ����ʽ������ۺϿ����⣬���������غ㶨�ɣ�������Һ�������仯�ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
14����ѧ��Դ��������������в��������ǣ�������
| A�� | ����ʱ���Ż��������Ϲ��� | |
| B�� | �������õĵؽ�ǰ�Ƚ��еƻ�ʵ�� | |
| C�� | �ü�ȩ��Һ����ʳ�ú���Ʒ�Ա��� | |
| D�� | �����ղ�����ζ�ķ���������֯��ʹ�ë֯�� |
1��ѧϰ��CO2���й�֪ʶ��ͬѧ�Dz������Ϸ���Mg����CO2��ȼ�գ���Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$��X+C����������X������˵����ȷ���ǣ�������
| A�� | ������һ���������� | |
| B�� | �����ʵĻ�ѧʽΪMgCO3 | |
| C�� | ��������þ����Ԫ�ص�������Ϊ 1��1 | |
| D�� | ����������Ԫ�ص�����������25% |
18������ʵ�������ȷ���ǣ�������
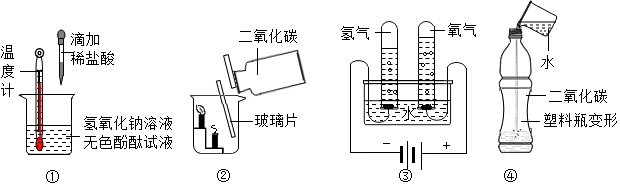
�ټ���˵���������кͷ�Ӧ������˵���кͷ�Ӧ�Ƿ��ȷ�Ӧ
�ڼ���˵��������̼���ܶȱȿ���������˵��������̼����ȼ��Ҳ��֧��ȼ��
�ۼ���˵��ˮ����Ԫ�ء���Ԫ����ɣ�����˵�����ɵ������������������Ϊ2��1
�ܼ���˵��������̼����ˮ������˵��������̼��һ���ᣮ

�ټ���˵���������кͷ�Ӧ������˵���кͷ�Ӧ�Ƿ��ȷ�Ӧ
�ڼ���˵��������̼���ܶȱȿ���������˵��������̼����ȼ��Ҳ��֧��ȼ��
�ۼ���˵��ˮ����Ԫ�ء���Ԫ����ɣ�����˵�����ɵ������������������Ϊ2��1
�ܼ���˵��������̼����ˮ������˵��������̼��һ���ᣮ
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
15���û�ѧ������գ�
������������2Fe3+������������Ԫ�صĻ��ϼ�H2$\stackrel{+6}{S}$O4����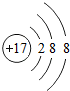 ��ʾ����Cl-��
��ʾ����Cl-��
��2�������м����������Ҵ���Ϊ����ȼ�ϣ����ʵ���ʡʯ����Դ����������Ⱦ��д���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽC2H5OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+3H2O��
��3����һ���ܱ���������A��B��C��D�������ʣ���һ�������³�ַ�Ӧ������������£�
��Ӧ������B��������2.2�ˣ��÷�Ӧ�����Ļ�����Ӧ�����ǻ��Ϸ�Ӧ��
��4��������������ͭ��ϡ���ᡢ������þ������������Һ������ͭ��Һ�������ʣ���������ܷ����ķ�Ӧ��6����
������������2Fe3+������������Ԫ�صĻ��ϼ�H2$\stackrel{+6}{S}$O4����
 ��ʾ����Cl-��
��ʾ����Cl-����2�������м����������Ҵ���Ϊ����ȼ�ϣ����ʵ���ʡʯ����Դ����������Ⱦ��д���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽC2H5OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+3H2O��
��3����һ���ܱ���������A��B��C��D�������ʣ���һ�������³�ַ�Ӧ������������£�
| ���� | A | B | C | D |
| ��Ӧǰ������/g | 6.4 | 3.2 | 4.0 | 2.8 |
| ��Ӧ�������/g | 5.2 | ���� | 7.2 | 2.0 |
��4��������������ͭ��ϡ���ᡢ������þ������������Һ������ͭ��Һ�������ʣ���������ܷ����ķ�Ӧ��6����