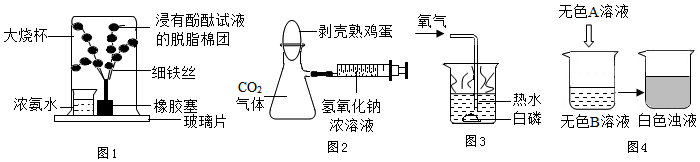
��Ŀ����
1����ѧԴ������������̺������ѧ֪ʶ����1����ϴ�ྫ��ϴ���ۣ���������ϴ�ྫ���黯���ã�
��2�����溬�е����ʡ����༰ά���ص�Ӫ���أ��������������³´�л��Ԥ���������õ���ά���أ�
��3�������괦�����������ڣ�ΪԤ�������Ͳ������Զ������Ʒ��ϺƤ��ʳ�����������Ҫ�ĸ�Ԫ�أ�
��4�������н�Ӳˮת��Ϊ��ˮ���õķ�������У�
��5������ʱ���е��Ͳ����Ż𣬿��ù��Ǹ��������ԭ��Ϊ����������
��6��д��һ��������ȡ�ĵ�̼����ľٴ������������
��7��д�����г������ķ����ʩͿ�ͣ�
���� ��1������ϴ�ྫ�����黯���÷�����
��2������ά���ص��������÷����ش�
��3�����ݻ�ѧԪ�������彡���Ĺ�ϵ��������
��4������Ӳˮ�����ķ������
��5���������ԭ�����
��6�����ݵ�̼������������
��7�����ݷ�ֹ������ķ������
��� �⣺��1����ϴ�ྫ��ϴ���ۣ���������ϴ�ྫ���黯���ã�����黯��
��2�����溬�е����ʡ����༰ά���ص�Ӫ���أ��������������³´�л��Ԥ���������õ�Ӫ������ά���أ����ά���أ�
��3��������ȱ�������Ͳ�������ƣ�
��4��Ӳˮ����ˮ�����п�����з�����
��5������ʱ���е��Ͳ����Ż𣬿��ù��Ǹ��������ԭ��Ϊ����������
��6���ᳫ����̼��������о������С�����������������һ���Կ��ӣ���Լֽ�ŵȣ�
��7�����г������ķ����ʩͿ�ͣ�
�𰸣���1���黯����2��ά���أ���3���ƣ���4����У���5��������������6����������������7��Ϳ�ͣ�
���� ���⿼������������ϵ���еĻ�ѧ֪ʶ����ѧ����ữѧ��Դ����������������ѧ�����ǵ�����ϢϢ��أ���������������ص�֪ʶ���п�������ȵ�֮һ�����������ѧ֪ʶ����ȷ�����Ĺؼ���


| A�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�� | B�� | CO+CuO$\frac{\underline{\;����\;}}{\;}$Cu+CO2 | ||
| C�� | CaO+H2O�TCa��OH��2 | D�� | 2NaOH+H2SO4=Na2SO4+2H2O |
| A�� | ��������ĩͶ��ʢ�й���������ձ��У���ַ�Ӧ�����к�ɫ���� | |
| B�� | ���ڿ�����ȼ�շ�������ɫ�Ļ��� | |
| C�� | ��ͥСʵ���У���ʳ���봿�����д������ݲ��� | |
| D�� | ��˿��������ȼ�շ����⣬���ɺ�ɫ���� |
| A�� | ������������������ | |
| B�� | ��������̼����Ԫ�ص�������Ϊ3��1 | |
| C�� | �����ữѧʽ��x=7 | |
| D�� | ÿ������������к���һ���������� |
 JD Pizza�dzԻ���ϲ���Ŀ����ʳ֮һ�����������Ӫ���ḻ�����еĵع����������ܵ����ǵ���������������������ϼ���عϡ��ཷ��ԭ�Ϻ濾�Ƶã���ش���������
JD Pizza�dzԻ���ϲ���Ŀ����ʳ֮һ�����������Ӫ���ḻ�����еĵع����������ܵ����ǵ���������������������ϼ���عϡ��ཷ��ԭ�Ϻ濾�Ƶã���ش���������