��Ŀ����
�綯���г���С�����Ƚ�ͨ�����ж���Ϊ���ṩ���ܵ�Ǧ���أ��ֳơ���ƿ�����������ŵ��ǿ��Գ��ѭ��ʹ�ã���ƿ�ڷ����Ļ�ѧ��Ӧ�ǣ�PbO2���̣�+2H2SO4+Pb=2PbSO4��+2H2Oij��ƿ��װ��36%��ϡ����1200g����ƿ����ʱ��310.5g��Ǧ�μӷ�Ӧ���Լ��㣺
��1��ԭϡ�������������ʵ������� g��
��2����ƿ����ʱ���������������
��3����Ӧ��������Һ�����ʵ��������������������ȷ��0.01%��
��1��ԭϡ�������������ʵ�������
��2����ƿ����ʱ���������������
��3����Ӧ��������Һ�����ʵ��������������������ȷ��0.01%��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���
��������1�����ʵ�����=��Һ�����������ʵ�����������
��2����Ǧ�������ͷ���ʽ��������ĵ��������ʵ�������
��3���ݷ���ʽ���������ˮ������������ʣ�����Һ����������������ʵ����������������Ӧ��������Һ�����ʵ�����������
��2����Ǧ�������ͷ���ʽ��������ĵ��������ʵ�������
��3���ݷ���ʽ���������ˮ������������ʣ�����Һ����������������ʵ����������������Ӧ��������Һ�����ʵ�����������
����⣺��1��ԭ��ϡ�������������ʵ�����=1200g��36%=432 g
��2�����������������ʵ�����Ϊx��ͬʱ����ˮ������Ϊy��
PbO2 ���̣�+2H2SO4+Pb=2PbSO4��+2H2O
196 207 36
x 310.5g y
=
x=294g
=
y=54g
��3��ʣ����Һ���������ʵ�����=432g-294g=138g
ʣ����Һ������=1200g-294g+54g=960g
��ʣ��������Һ�����ʵ���������=
x 100%=14.38%
�ʴ�Ϊ����1��432����2��������ϡ����������Ϊ294g����3����ʱ��ƿ��������Һ�����ʵ�����������14.38%��
��2�����������������ʵ�����Ϊx��ͬʱ����ˮ������Ϊy��
PbO2 ���̣�+2H2SO4+Pb=2PbSO4��+2H2O
196 207 36
x 310.5g y
| 196 |
| x |
| 207 |
| 310.5g |
x=294g
| 207 |
| 310.5g |
| 36 |
| y |
y=54g
��3��ʣ����Һ���������ʵ�����=432g-294g=138g
ʣ����Һ������=1200g-294g+54g=960g
��ʣ��������Һ�����ʵ���������=
| 138g |
| 960g |
�ʴ�Ϊ����1��432����2��������ϡ����������Ϊ294g����3����ʱ��ƿ��������Һ�����ʵ�����������14.38%��
�����������ʱ��Ҫע�⣬��Ӧֻ��������Ǧ������ˮ�����Է�Ӧ��ɺ�������ҺΪʣ��ϡ���������������ˮ�������ͣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ˮ����Һ������������������ʮ����Ҫ�����ã�
ˮ����Һ������������������ʮ����Ҫ�����ã�




 ��1��Cd��OH��2��Cd��OԪ��������Ϊ
��1��Cd��OH��2��Cd��OԪ��������Ϊ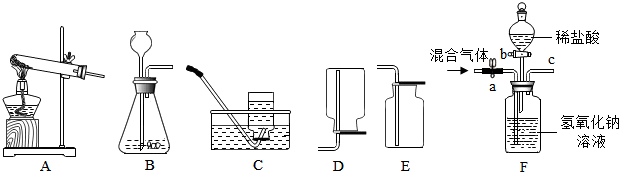
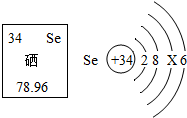 �����г������ۡ������ʡ�����ά�����⣬�����������غ���Ԫ�أ�������Ԫ�صIJ�����Ϣ��ͼ��ʾ��
�����г������ۡ������ʡ�����ά�����⣬�����������غ���Ԫ�أ�������Ԫ�صIJ�����Ϣ��ͼ��ʾ��