��Ŀ����
12��ɽ����Ȫ�¾�����������˹��ը�¹ʣ����3�������������ˣ���˹������ú�㼰ú��Χ����ʯ���У���ú�����к�������ܳƣ��ǵ���ú���¹ʵġ�����ɱ�֡�����1����˹���ڻ���� �����������������
��2��ú�������˹��ըҪ����������������˹Ũ��Ҫ�ﵽ��ը���ޡ��㹻�����������𣻿��ƺ����е����������Ա��ⱬը�¹ʵķ�������������ѧ֪ʶ�Ծ�һ������˹��ը�¹ʷ�������Ч��ʩ����ֹ�̻�
���� ��1���������ʵķ��������
��2��������˹��ը��������������˹��ը����Ч��ʩ������
��� �⣺��1��������˹�Ĵ��ڻ�������ú�����к�������ܳƿ����жϳ�����Ӧ���ǻ����ʱ����Ϊ������
��2����˹����Ҫ�ɷ��Ǽ��飬����˹�������ϴﵽ����ı�ը����ʱ��������ᷢ����ը����˱�����˹��ը��Ӧ�ý�ֹ�̻𣬾���ͨ�绻�����Է�ֹ��ȫ�¹ʵķ������ʴ�Ϊ�����������𣻽�ֹ�̻�
���� ���⿼�������ʵķ��࣬��ȼ�ױ��﷽���֪ʶ��ѧ������ͻ�������ʱ������ұ�����

��ϰ��ϵ�д�
�����Ŀ
20��ʳ������ζ������Ϊʳ���к��д��ᣨ��ѧʽΪCH3COOH�������и������ƵĻ�ѧ���ʣ������йش��ữѧ���ʵ�����������ǣ�������
| A�� | �����ͭ��Ӧ������������ | B�� | �����ⷴӦ����ˮ���� | ||
| C�� | ���ռӦ����ˮ���� | D�� | �뼦���Ƿ�Ӧ������������ |
17��ij��ȤС���ͬѧ���֣��ϸ�����ʵ���õ�����������Һ����������ƿ������ƿ��Һ��û�б����أ�ͬѧ����һ̽���������������һ����룮
���������ϡ��Ȼ�����Һ�����ԣ����Ȼ�����̼�����ܷ�Ӧ����̼�ᱵ��ɫ�������Ȼ��ƣ�
��������롿����һ������Һû�б��ʣ�
�����������Һ���ֱ��ʣ�
������������Һȫ�����ʣ�
��ʵ����֤��
�����۽�����
��1������ٵμӹ������Ȼ�����Һ��Ŀ���dz�����Һ�е�̼���ƣ�����Լ�������������ɸ��ţ�
��2����ͬѧ���������������Һ�����Ȼ�����Һ��ͬ����ʵ�飬Ҳ�ܿ�����ͬ�����ó���ͬ�Ľ��ۣ���ͬ�����Ĺ۵���Ϊʲô����ͬ�⣬��Ϊ��������������Һ������OH-���Լ����������ƻ���ɸ��ţ�
����˼���������Ƴ��ڷ��ñ��ʵ�ԭ����2NaOH+CO2�TNa2CO3+H2O���û�ѧ����ʽ��ʾ���������������Ӧ�ܷⱣ�森
�������о������ԭ��������C-12 O-16 Na-23 Ca-40 Cl-35.5
�Ӹ�ƿ�Ѳ��ֱ���Ϊ̼���Ƶ�����������Һ��ȡ��100.0g��Һ�������м���100.0g�Ȼ�����Һ����ȫ��Ӧ����ˣ������Һ������Ϊ190.0g��
����������ȷ��С�����һλ���йػ�ѧ����ʽ��Na2CO3+CaCl2=CaCO3+2NaCl��
��1����Ӧ���ɵij���������10g��
��2����ȡ����Һ�к�̼���Ƶ������Ƕ��ٿˣ�
�� 3����Ӧ����Һ���Ȼ������ʵ�����������
���������ϡ��Ȼ�����Һ�����ԣ����Ȼ�����̼�����ܷ�Ӧ����̼�ᱵ��ɫ�������Ȼ��ƣ�
��������롿����һ������Һû�б��ʣ�
�����������Һ���ֱ��ʣ�
������������Һȫ�����ʣ�
��ʵ����֤��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ��������Һ���Թ��У������еμӹ������Ȼ�����Һ�� | ���ɰ�ɫ���� | ����һ������ |
| �ھ��ã����ϲ���Һ�еμӷ�̪��Һ�� | ��Һ��� | ��������� |
��1������ٵμӹ������Ȼ�����Һ��Ŀ���dz�����Һ�е�̼���ƣ�����Լ�������������ɸ��ţ�
��2����ͬѧ���������������Һ�����Ȼ�����Һ��ͬ����ʵ�飬Ҳ�ܿ�����ͬ�����ó���ͬ�Ľ��ۣ���ͬ�����Ĺ۵���Ϊʲô����ͬ�⣬��Ϊ��������������Һ������OH-���Լ����������ƻ���ɸ��ţ�
����˼���������Ƴ��ڷ��ñ��ʵ�ԭ����2NaOH+CO2�TNa2CO3+H2O���û�ѧ����ʽ��ʾ���������������Ӧ�ܷⱣ�森
�������о������ԭ��������C-12 O-16 Na-23 Ca-40 Cl-35.5
�Ӹ�ƿ�Ѳ��ֱ���Ϊ̼���Ƶ�����������Һ��ȡ��100.0g��Һ�������м���100.0g�Ȼ�����Һ����ȫ��Ӧ����ˣ������Һ������Ϊ190.0g��
����������ȷ��С�����һλ���йػ�ѧ����ʽ��Na2CO3+CaCl2=CaCO3+2NaCl��
��1����Ӧ���ɵij���������10g��
��2����ȡ����Һ�к�̼���Ƶ������Ƕ��ٿˣ�
�� 3����Ӧ����Һ���Ȼ������ʵ�����������
4����ѧ�����ǵ������������еĹ�ϵ������˵������ȷ���ǣ�������
| A�� | ��ù�Ļƶ�ϴ�ɾ�����Ƴɶ���ʳ�� | |
| B�� | ��ʳ�߲�ˮ����ͬѧ��ȱ��ά����C�����ܻ�ҹä֢ | |
| C�� | ����ȱ��������ƶѪ�����Ӧ�������� | |
| D�� | ���������������������ڣ�Ӧ��ʳ�ú������ʷḻ��ʳ�� |
1�����к���Ԫ�ص������У���Ԫ�صĻ��ϼ��ɵ͵��ߵ�һ���ǣ�������
| A�� | Cl2��CaCl2��NaClO | B�� | CaCl2��NaClO��Cl2 | C�� | NaClO��Cl2��CaCl2 | D�� | CaCl2��Cl2��NaClO |
4����ͼΪ�ס�����������ܽ�����ߣ�����˵������ȷ���ǣ�������


| A�� | tl��ʱ��100g����Һ�к������ʵ�����С��30g | |
| B�� | tl��ʱ�����ҵ��ܽ����ͬ | |
| C�� | t2��ʱ���������ļס��ҵı�����Һ���µ�tl��ʱ��������״����� | |
| D�� | t2��ʱ���ı�����Һ������������Ϊ50% |
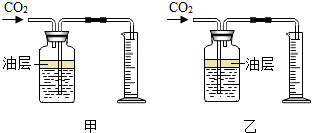 ��ѧ��ȤС��ⶨijʯ��ʯ��Ʒ��̼��Ƶ���������
��ѧ��ȤС��ⶨijʯ��ʯ��Ʒ��̼��Ƶ���������