��Ŀ����
14������һ���������ʵ�BaCl2������Ʒ�����ʲ�����ˮ����Ϊ���о���Ʒ����ɣ��������������飬��ش��������⣺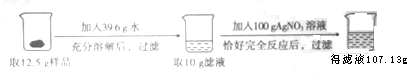
��1��ʵ�鷢����Ӧ�Ļ�ѧ����ʽΪBaCl2+2AgNO3=2AgCl��+Ba��NO3��2��
��2���г����10g��Һ������������x���ı���ʽ$\frac{208}{x}$=$\frac{287}{2.87g}$��
��3��AgNO3��Һ��������������Ϊ3.4%��
��4�������ˮ����ܽ��������Һ��������������Ϊ20.8%��
��5��������������Һ�Ĺ����У�������˵����Һ�����ʵı仯������������
��6����Ʒ��BaCl2�ĺ���Ϊ10.4g��
���� ���������غ㶨�ɿ�֪��10g��Һ��100g��������Һ���ǰ����Һ�������������Ϊ�������������Ȼ��������Կ��������Ȼ����������������Ȼ����Ͷ�Ӧ�Ļ�ѧ����ʽ�����Ȼ����������������ᱵ��������Ҫ����ԭ������е��Ȼ��Ƶģ�����������������Ӧ�����ʵ�����������Ӧ�����忼�Ǻ�һ�����㣮
��� �⣺�����ķ�ӦΪBaCl2+2AgNO3=2AgCl��+Ba��NO3��2����
���������غ㶨�ɿ�֪��10g��Һ��100g��������Һ���ǰ����Һ�������������Ϊ�������������Ȼ��������Կ��������Ȼ���������10g+100g-107.13g=2.87g
��10g��Һ���Ȼ���������Ϊx��100g��������Һ��������������Ϊy�����ɵ����ᱵ������Ϊz��
BaCl2+2AgNO3=2AgCl��+Ba��NO3��2����
208 340 287 261
x y 2.87g z
$\frac{208}{x}$=$\frac{340}{y}$=$\frac{287}{2.87g}$=$\frac{261}{z}$
x=2.08g
y=3.4g
z=2.61g
��������Һ������������������Ϊ$\frac{3.4g}{100g}$��100%=34%��
��������ˮ��õ�����Һ��������������ȡ����10g ��Һ����ͬ����Һ���о�һ�ԣ�������������Ϊ$\frac{2.08g}{10g}$��100%=20.8%
������������Һ�Ĺ����У�������˵����Һ�����ʵı仯��������������Ȼ�����Ϊ���ᱵ�����������ڷ�Ӧ�е�������ϵ��208��261������֪�����ʵ����������ӣ�
����Ʒ�е��Ȼ���������Ϊa��
������Һ�ľ�һ�ԣ���������ܽ��������Һ����������Ϊ20.8%
Ϊ���㷽�㣬��ˮ����������Ϊ1-20.8%=79.2%
��$\frac{39.6g}{a+39.6g}$��100%=79.2%
a=10.4g
�𣺣�1��ʵ�鷢����Ӧ�Ļ�ѧ����ʽΪ BaCl2+2AgNO3=2AgCl��+Ba��NO3��2��
��2���г����10g��Һ������������x���ı���ʽ $\frac{208}{x}$=$\frac{287}{2.87g}$��
��3��AgNO3��Һ��������������Ϊ 3.4%��
��4�������ˮ����ܽ��������Һ��������������Ϊ 20.8%��
��5��������������Һ�Ĺ����У�������˵����Һ�����ʵı仯����� �������
��6����Ʒ��BaCl2�ĺ���Ϊ 10.4g��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| A�� | ��Ӧǰ��ԭ�����ࡢ��Ŀ�ı� | B�� | �÷�Ӧ�����û���Ӧ | ||
| C�� | �÷�Ӧ�У�MΪCO2 | D�� | �÷�Ӧ�У���Ӧ�����������1��4 |
| A�� | ��ĭ���ϡ������ǿ�ȼ�� | |
| B�� | ��ĭ���ϡ�������Ż��ܸ� | |
| C�� | ȼ�ղ�����Ũ���к��д����ж����� | |
| D�� | �Ż�ʱӦ����ʪ����ס�ڱǣ�����ȫ�� |
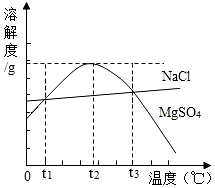 ��ͼ��NaCl��MgSO4���ܽ�����ߣ�����˵����ȷ���ǣ�������
��ͼ��NaCl��MgSO4���ܽ�����ߣ�����˵����ȷ���ǣ�������| A�� | ֻ����t1��ʱ��NaCl��MgSO4���ܽ�Ȳ���� | |
| B�� | MgSO4�ı�����Һ��t2��ʱ�����ʵ������������ | |
| C�� | t1�桫t3�棬MgSO4���ܽ�����¶����߶����� | |
| D�� | t3��ʱ��MgSO4������Һ������t2��ʱ���о������� |
| A�� | ������ú��й©��������ͨ�� | B�� | ������úȡůʱ����Ŵ� | ||
| C�� | ��úҤ����ú�û������ | D�� | ���е����Ż��ù��Ǹ��� |
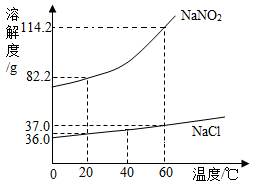 �������ƣ�NaNO2���ж�������ζ��������ʳ�����ƣ�������ʳ�������ж���NaNO2��NaCl���ܽ��������ͼ��ʾ��
�������ƣ�NaNO2���ж�������ζ��������ʳ�����ƣ�������ʳ�������ж���NaNO2��NaCl���ܽ��������ͼ��ʾ��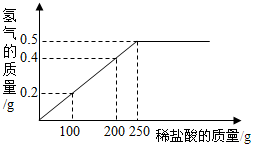 ����ͭ�ۺ�þ�۵Ļ������Ʒ��ij��ȤС��Ҫ�ⶨ��Ʒ��þ���������������dz�ȡ�û������Ʒ10g�����ձ��У�Ȼ�����350gһ������������ϡ���ᣮ����ϡ��������������������������ϵ��ͼ��ʾ������㣺
����ͭ�ۺ�þ�۵Ļ������Ʒ��ij��ȤС��Ҫ�ⶨ��Ʒ��þ���������������dz�ȡ�û������Ʒ10g�����ձ��У�Ȼ�����350gһ������������ϡ���ᣮ����ϡ��������������������������ϵ��ͼ��ʾ������㣺