��Ŀ����
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�һ��������������Ϊ��̼������Һ����¼����ͼ��ʾ�����߹�ϵ������Ա��ʾС�����Ȼ�����̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��CaCl2+Na2CO3 ===CaCO3��+2NaCl����
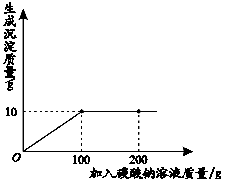
��1�����Ȼ�����̼����ǡ����ȫ��Ӧʱ������̼������Һ�������� ��
��2��������Ʒ���Ȼ��Ƶ�������
��3�����Ȼ�����̼����ǡ����ȫ��Ӧʱ�����ˣ�������Һ�����ʵ����������Ƕ��٣�?
��2��������Ʒ���Ȼ��Ƶ�������
��3�����Ȼ�����̼����ǡ����ȫ��Ӧʱ�����ˣ�������Һ�����ʵ����������Ƕ��٣�?
(1)100 g
(2)�⣺����Ʒ���Ȼ��Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy??
CaCl2+Na2CO3===CaCO3��+2NaCl?
111 ? 100 117 ??
x? 10g y
111��x= 100��10g ?x=11.1g
117��y = 100��10g ?y=11.7g ?
��Ʒ���Ȼ��Ƶ�����=13.4g-11.1g=2.3g ?
(3)������Һ��������������=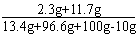 ��100%=7%
��100%=7%
(2)�⣺����Ʒ���Ȼ��Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy??
CaCl2+Na2CO3===CaCO3��+2NaCl?
111 ? 100 117 ??
x? 10g y
111��x= 100��10g ?x=11.1g
117��y = 100��10g ?y=11.7g ?
��Ʒ���Ȼ��Ƶ�����=13.4g-11.1g=2.3g ?
(3)������Һ��������������=
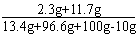 ��100%=7%
��100%=7% 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ��
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ�� ��2013?������ģ�⣩С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ��
��2013?������ģ�⣩С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ�� С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼������ͼ��ʾ�����߹�ϵ������Ա��ʾС�����Ȼ�����̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��CaCl2+Na2CO3=CaCO3��+2NaCl��
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼������ͼ��ʾ�����߹�ϵ������Ա��ʾС�����Ȼ�����̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��CaCl2+Na2CO3=CaCO3��+2NaCl�� С��ͬѧ��ij�������������ʵ��������Ա��С��һ�����ij�Ȼ�����Ʒ�����������Ȼ������ʣ�����ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ�����ó������������̼������Һ��������ͼ����ش�
С��ͬѧ��ij�������������ʵ��������Ա��С��һ�����ij�Ȼ�����Ʒ�����������Ȼ������ʣ�����ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ�����ó������������̼������Һ��������ͼ����ش� С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼��ͼ��ʾ�����߹�ϵ����
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ��ƺ��Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶĺ�������ȡ13.4g������Ʒ��ȫ������96.6gˮ�У������õĻ����Һ�еμ�������������Ϊ10.6%��̼������Һ����¼��ͼ��ʾ�����߹�ϵ����