��Ŀ����
Ϊ�ⶨͭп�Ͻ��и��ɷֵ���ɣ�ȡ20gͭп�Ͻ���Ʒ����һ����������������120gϡ�����6�μ�����Ʒ�У�ÿ�γ�ַ�Ӧ���ȡ�����壬�����ˡ�����Ȳ���������ÿ��ʣ������������ϡ�����������¼���±���
����㣺
��1���ϱ��пո��ڵ�����Ϊ ��
��2��ͭп�Ͻ���Ʒ��п������������
��3������ϡ���������ʵ�����������
| ʵ���� | ϡ���������/g | ʣ����������/g |
| 1 | 20 | 17.4 |
| 2 | 20 | 12.2 |
| 3 | 20 | 9.6 |
| 4 | 20 | 7 |
| 5 | 20 | 7 |
| 6 | 20 |
��1���ϱ��пո��ڵ�����Ϊ
��2��ͭп�Ͻ���Ʒ��п������������
��3������ϡ���������ʵ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1������ͭ�������ᷴӦ����4�μ������ᷴӦ�����ʣ���7g����ijɷ���ͭ���з�����
��2�����ݷ�Ӧǰ��������ʼ��ٵ�����Ϊп���������������������ļ��㹫ʽ�������пͭ�Ͻ���п������������
��3������20g����ֻ������2.6gп���м��㣬��������������ٳ���20g���ٷ�֮�ټ��ɽ��
��2�����ݷ�Ӧǰ��������ʼ��ٵ�����Ϊп���������������������ļ��㹫ʽ�������пͭ�Ͻ���п������������
��3������20g����ֻ������2.6gп���м��㣬��������������ٳ���20g���ٷ�֮�ټ��ɽ��
����⣺��1��ͭ�������ᷴӦ����4�μ������ᷴӦ�����ʣ���7g����ijɷ���ͭ�������ϱ��пո��ڵ�����Ϊ7��
��2����ϣ�1���Ľ�����������ݿ���֪������μ���ϡ��������������û�иı䣬���Կ����ж�ͭ������Ϊ7g��
����п����������Ϊ=
��100%=65%��
���ԺϽ���Ʒ��п����������Ϊ65%��
��3����20��ϡ����������H2SO4������Ϊx��
Zn+H2SO4=ZnSO4+H2��
65 98
2.6g x
=
x=3.92g
����ϡ���������ʵ�����������
��100=19.6%
������ϡ���������ʵ���������Ϊ19.6%��
�ʴ�Ϊ����1��7��
��2��65%��
��3��19.6%��
��2����ϣ�1���Ľ�����������ݿ���֪������μ���ϡ��������������û�иı䣬���Կ����ж�ͭ������Ϊ7g��
����п����������Ϊ=
| 20-7 |
| 20 |
���ԺϽ���Ʒ��п����������Ϊ65%��
��3����20��ϡ����������H2SO4������Ϊx��
Zn+H2SO4=ZnSO4+H2��
65 98
2.6g x
| 65 |
| 2.6g |
| 98 |
| x |
x=3.92g
����ϡ���������ʵ�����������
| 3.92 |
| 20 |
������ϡ���������ʵ���������Ϊ19.6%��
�ʴ�Ϊ����1��7��
��2��65%��
��3��19.6%��
�����������㿼���˱����ͼ����⣬�������������ͻ�ѧ����ʽ���ۺ�Ӧ�ã����п��������о������ֵ����ͣ�����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨�ɣ���Ҫ���Ρ�������ֵҪ�����塱����������Ҫ�����ڼ������У�

��ϰ��ϵ�д�
 �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
�����Ŀ
���м������ʱ�¶�ڿ����У�ǰ���������Ӳ����ʣ����߱���������Ҳ���ӵ��ǣ�������
| A��H2SO4 NaOH |
| B��NaOH Ca��OH��2 |
| C��ŨH2SO4 CaO |
| D��Ca��OH��2 CuSO4 |
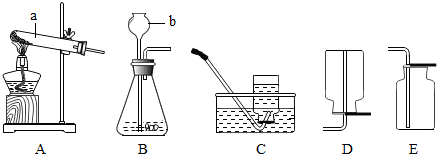
��ͼװ�ó������ⶨ�������������������жԸ�ʵ�����ʶ����ȷ���ǣ�������


| A������ȼ�ղ���������ɫ���� | ||
B������������ᵼ�½��뼯��ƿ��ˮ���������
| ||
| C����ʵ���˵��N2������ˮ | ||
| D��ȼ�ճ��еĺ����Ի���ϸ��˿ |
�����й�ȼ�ա���ը�����˵����ȷ���ǣ�������
| A��������������棬��Ϊ���������� |
| B����ѹ������ѹ�����������ı�ը�ǻ�ѧ�仯 |
| C������Ա�ø�ѹˮǹ�����Ϊ�����˿�ȼ����Ż�� |
| D������ɭ�ֻ���ʱ�����ٸ��������Ŀ���Ǹ����ȼ�� |