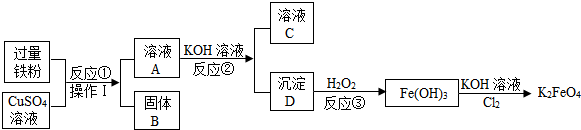
��Ŀ����
ijУ��ѧ�о�С���ͬѧͨ��ʵ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ10g��Ʒ�����Ĵμ���ϡ���ᣬ���ÿ�����ɶ�����̼�����������ʾ�����ʲ������ᷴӦ��
��1��̼����У��ơ�̼����Ԫ�ص�������Ϊ ��
��2������m= ��10g��Ʒ���Ĵμ���ϡ���������CO2������Ϊ ��
��3���Լ����ʯ��ʯ��Ʒ��̼��Ƶ�����������
| ʵ�� | 1 | 2 | 3 | 4 |
| ����ϡ����/mL | 15 | 15 | 15 | 15 |
| ������̼����/g | 1.50 | 1.50 | 0.52 | m |
��2������m=
��3���Լ����ʯ��ʯ��Ʒ��̼��Ƶ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����ݻ������и�Ԫ�ص��������������ԭ������֮�͵ıȽ��н��
��2�����ݼ�¼���ݿɷ��֣��ڶ���ʵ���м������ỹ�ж�����̼�ų���˵����һ��ʵ����������ȫ����Ӧ���ų�������̼������Ϊ1.50g��������ʵ����ֻ�ų�0.52g������̼��˵��������ʣ�࣬�����ڵ�����ʵ����̼����Ѿ���Ӧ�꣬���Ե��Ĵ��ټ���������ж�����̼����ų����н��
��3�����ݶ�����̼�����������ʯ��ʯ��Ʒ��̼��Ƶ����������������ʯ��ʯ��Ʒ��̼��Ƶ������������ɣ�
��2�����ݼ�¼���ݿɷ��֣��ڶ���ʵ���м������ỹ�ж�����̼�ų���˵����һ��ʵ����������ȫ����Ӧ���ų�������̼������Ϊ1.50g��������ʵ����ֻ�ų�0.52g������̼��˵��������ʣ�࣬�����ڵ�����ʵ����̼����Ѿ���Ӧ�꣬���Ե��Ĵ��ټ���������ж�����̼����ų����н��
��3�����ݶ�����̼�����������ʯ��ʯ��Ʒ��̼��Ƶ����������������ʯ��ʯ��Ʒ��̼��Ƶ������������ɣ�
����⣺��1��̼����У��ơ�̼����Ԫ�ص�������=40��12����16��3��=10��3��12�����10��3��12��
��2�����ݼ�¼���ݿɷ��֣��ڶ���ʵ���м������ỹ�ж�����̼�ų���˵����һ��ʵ����������ȫ����Ӧ���ų�������̼������Ϊ1.50g��������ʵ����ֻ�ų�0.52g������̼��˵��������ʣ�࣬�����ڵ�����ʵ����̼����Ѿ���Ӧ�꣬���Ե��Ĵ��ټ���������ж�����̼����ų�������m��ֵΪ0��������CO2������=1.50g+1.50g+0.52g=3.52g�����0��3.52g��
��3����ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.52g
=
x=8g
ʯ��ʯ��Ʒ��CaCO3����������=
��100%=80%
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ80%��
��2�����ݼ�¼���ݿɷ��֣��ڶ���ʵ���м������ỹ�ж�����̼�ų���˵����һ��ʵ����������ȫ����Ӧ���ų�������̼������Ϊ1.50g��������ʵ����ֻ�ų�0.52g������̼��˵��������ʣ�࣬�����ڵ�����ʵ����̼����Ѿ���Ӧ�꣬���Ե��Ĵ��ټ���������ж�����̼����ų�������m��ֵΪ0��������CO2������=1.50g+1.50g+0.52g=3.52g�����0��3.52g��
��3����ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.52g
| 100 |
| x |
| 44 |
| 3.52g |
x=8g
ʯ��ʯ��Ʒ��CaCO3����������=
| 8g |
| 10g |
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ80%��
������������Ҫ������ѧ������ͼ�����ݣ����������������Լ��ݷ���ʽ�����������

��ϰ��ϵ�д�
�����Ŀ
���л�ѧʵ�����ע�ⰲȫ��������������ʵ�ʵ��ǣ�������
| A��������Ũ����մ��Ƥ���ϣ�Ӧ������ϡ��ˮ��ϴ����Ϳ��������Һ |
| B������������ǿ�ҵĸ�ʴ�ԣ�ʵ��ʹ��ʱ����ô��Ϸ����۾� |
| C����ʵ���������Ʒ���İ취����ʳ�κ����Ǿ��� |
| D��ֱ�Ӱѱǿ״յ��Լ�ƿ����Ũ������ζ |
ʵ����������������ķ��÷�����ȷ���ǣ�������
A�� |
B�� |
C�� |
D�� |