��Ŀ����
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5 g������Ʒ������Ũ�ռ���Һ���ȣ������İ�����100.0g���������ա�������հ�������Һ������m�뷴Ӧʱ��t�ı仯������ͼ��ʾ�����漰�ķ�ӦΪ�� (NH4)2SO4+2NaOH Na2SO4+2H2O+2NH3���� 2NH3+H2SO4 == (NH4)2SO4 ��
Na2SO4+2H2O+2NH3���� 2NH3+H2SO4 == (NH4)2SO4 ��
 Na2SO4+2H2O+2NH3���� 2NH3+H2SO4 == (NH4)2SO4 ��
Na2SO4+2H2O+2NH3���� 2NH3+H2SO4 == (NH4)2SO4 �� 
����㣺
��1����ȫ��Ӧ��������� g��
��2���û��ʵĺ�����Ϊ ����ȷ��0.1%���������ֻ������� (����ϸ��ϸ��ϸ�����狀�����Ϊ20%����)��Ʒ��
��3�����������������������������д��������̣���
��4����ʵ������а�������ȫ���գ�����ʵ��������炙��ʵĺ���������ʵ��ֵ����ԭ���� ��
��1����ȫ��Ӧ��������� g��
��2���û��ʵĺ�����Ϊ ����ȷ��0.1%���������ֻ������� (����ϸ��ϸ��ϸ�����狀�����Ϊ20%����)��Ʒ��
��3�����������������������������д��������̣���
��4����ʵ������а�������ȫ���գ�����ʵ��������炙��ʵĺ���������ʵ��ֵ����ԭ���� ��
�⣺��1��6.8g
��2��20.4%���ϸ�
��3���⣺��������д����������Ϊx
2NH3 + H2SO4 == (NH4)2SO4
34 98
6.8g x

H2SO4��������=(19.6g��100.0g)��100%=19.6%
�𣺷��������������������Ϊ19.6%��
��4�����ȷ�Ӧ������ˮ���������������գ�������������Һ������ֻ���հ�����
��2��20.4%���ϸ�
��3���⣺��������д����������Ϊx
2NH3 + H2SO4 == (NH4)2SO4
34 98
6.8g x

H2SO4��������=(19.6g��100.0g)��100%=19.6%
�𣺷��������������������Ϊ19.6%��
��4�����ȷ�Ӧ������ˮ���������������գ�������������Һ������ֻ���հ�����

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
�����Ŀ
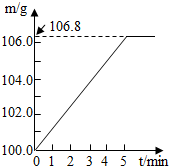 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺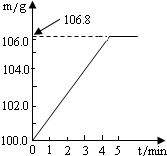
 ��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ���������
��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ��������� ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺