��Ŀ����
 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺��1��������Ʒ��ȫ��Ӧ���������
6.8
6.8
g����2���û��ʵĺ�����Ϊ
20.4%
20.4%
����ȷ��0.1%���������ϸ�
�ϸ�
��ѡ��ϸ��ϸ���Ʒ����3����Ӧ�������ĵ�����������Ƕ��٣�д��������̣���
���������������֪���ݷ�������Һ���հ����������仯������������������Ӷ��õ��������������ʵ��������Ϳ��Լ�����û��ʵĺ��������ж��Ƿ�Ϊ�ϸ��Ʒ�����������백���ķ�Ӧ�Ļ�ѧ����ʽ�ɼ���������������
����⣺��1�����������غ㶨�ɽ������ͼʾ��֪���ɵİ�������Ϊ106.8g-100g=6.8g���ʴ�Ϊ��6.8
��2�����������غ㶨�ɰ����еĵ�Ԫ������������炙����е�Ԫ�ص������������е�Ԫ�ص�����Ϊ6.8g��
=5.6g���ʻ����е�Ԫ�ص���������Ϊ
��100%=20.4%
�ʴ�Ϊ��20.4% �ϸ�
��3�������ĵ�H2SO4 ����Ϊx
H2SO4+2NH3=��NH4��2SO4
98 34
x 6.8 g
=
x=19.6g
�𣺷�Ӧ�������ĵ����������Ϊ19.6g��
��2�����������غ㶨�ɰ����еĵ�Ԫ������������炙����е�Ԫ�ص������������е�Ԫ�ص�����Ϊ6.8g��
| 14 |
| 17 |
| 5.6g |
| 27.5g |
�ʴ�Ϊ��20.4% �ϸ�
��3�������ĵ�H2SO4 ����Ϊx
H2SO4+2NH3=��NH4��2SO4
98 34
x 6.8 g
| 98 |
| x |
| 34 |
| 6.8g |
x=19.6g
�𣺷�Ӧ�������ĵ����������Ϊ19.6g��
�������������ۺ��Լ����⣬���������غ㶨�ɵļ��㡢���ݻ�ѧʽ�ļ�����������������ļ��㣬���ؼ��������Ŀ��飬ƽʱѧϰʱһ��ע��������ܽ���ɣ�Ѱ�ҽ���˼·��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
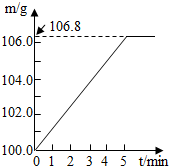 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺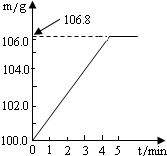
 ��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ���������
��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ���������