��Ŀ����
��Ӣͬѧȡij��ʯ��ʯ��Ʒ12g���вⶨʵ�飬�ֽ�100gϡ�������μ���ʯ��ʯ��Ʒ�У����ʲ�����ˮҲ�����뷴Ӧ������ַ�Ӧ������������������������ʾ��
| ��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
| ����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
| ���������������/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
����
��1��m��ֵΪ�� ��g��
��2��12gʯ��ʯ��Ʒ��̼��Ƶ����������� ��g��
��3����Ӧ��ȫ��������Һ���Ȼ��Ƶ������������� ����
��д��������̣���������ȷ��0.1��
���㣺
���ݻ�ѧ��Ӧ����ʽ�ļ��㣻�й��������������ļ��㣮
ר�⣺
�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩��
������
��1�����ݼ�¼���ݿɷ��֣�20g��������ȫ��Ӧ���ɶ�����̼�����������1.1g����4��ʵ���м����������ɵĶ�����̼��4.4g��˵���˵�����ʵ����������ȫ��Ӧ�����Ƴ�m��ֵ��
��2�����ݶ�����̼�����������ʯ��ʯ��Ʒ��̼��Ƶ����������ɵ��Ȼ��Ƶ�������
��3���������������������ʵ��������������Ӧ��ȫ��������Һ���Ȼ��Ƶ�����������
���
�⣺��1�����ݼ�¼���ݿɷ��֣�20g��������ȫ��Ӧ���ɶ�����̼�����������1.1g����4��ʵ���м����������ɵĶ�����̼��4.4g��˵���˵�����ʵ����������ȫ��Ӧ�����Ƴ�m��ֵΪ3.3g��
��2����ʯ��ʯ��CaCO3������Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
x y 4.4g

��ã�x=10g y=11.1g
��3����ȫ��Ӧ��������Һ���Ȼ��Ƶ����������ǣ�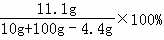 ��10.5%
��10.5%
�ʴ�Ϊ����1��3.3����2��10����3��10.5%��
������
������Ҫ������ѧ������ͼ�����ݣ����������������Լ��ݷ���ʽ�����������
��

��ʢ��5g 16%��NaOH��Һ�Ķ��Թܷ���ʢ��10g 16%��CuSO4��Һ����ƿ���ͼ��ʾ����б��ƿֱ��ʹ����Һ��ֻ�ϣ���Ϻ��ܵ��ǣ�������
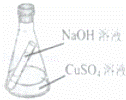
| �� | A�� | ������ɫ����0.98g |
| �� | B�� | ��ƿ���������������� |
| �� | C�� | ������Һ��Na2SO4������������9.5% |
| �� | D�� | ���õ�Na2SO4��Һ������ԭCuSO4��Һ������� |
��ѧ���϶���ʳ����������Ҫ���ã����������ʵ��ǣ�������
| �� | A�� | NH4HCO3 | B�� | CO��NH2��2 | C�� | KNO3 | D�� | Ca��H2PO4��2 |
