��Ŀ����
3��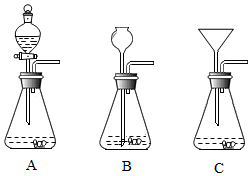 ij��ȤС��Ϊ�ⶨʯ��ʯ��Ʒ��̼��Ƶ���������������ʯ��ʯ��ϡ���ᷴӦ��ԭ�����вⶨ��������ش�
ij��ȤС��Ϊ�ⶨʯ��ʯ��Ʒ��̼��Ƶ���������������ʯ��ʯ��ϡ���ᷴӦ��ԭ�����вⶨ��������ش���1����С��ͬѧ�����ṩ��ʵ�������ֱ����������������ȡ������̼��װ�ã����Ƕ���Ƶ�װ�ý����˽������ۣ���������Ϊ���巢��װ�õ���AB������ĸ��ţ�
��2����ͬѧȡ��10����Ʒ��������ϡ���ᣨ���ʲ���ϡ���ᷴӦ����Ӧ��ʵ�鹲�ռ���3.3�˶�����̼�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3����ͬѧ��Ϊ�����˲ⶨ������CO2���������ⶨ��Ʒ��̼��������������⣬����������¼��ַ���������Ϊ���е���AB������ţ���
A���ȳ�ȡ��Ʒ����Ϊx�ˣ��ٳ�ȡ10%������y�ˣ�����Ʒ�еμ���������Ʒǡ����ȫ��Ӧ����ȡʣ����������Ϊz�ˣ�����x��y��z����̼�������������
B���ȳ�ȡ��Ʒ����Ϊx�ˣ��ٵμ�ϡ��������Ʒǡ����ȫ��Ӧ���ٽ�����Һ����ˡ��������ᾧ����ɣ��Ƶù�������Ϊy�ˣ�����x��y����̼�������������
C���ȳ�ȡ��Ʒ����Ϊx�ˣ��ٵμ�ϡ��������Ʒǡ����ȫ��Ӧ���ٲⶨ����Һ����ˮ������Ϊy�ˣ�����x��y����̼�������������
���� ̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ݷ�Ӧ�Ļ�ѧ����ʽ�����ṩ�����ݿ��Խ�����ط���ļ�����жϣ�
��� �⣺��1����������Ϊ���巢��װ�õ���AB��C������������װ�õ�ԭ���Ƿ�Ӧ���ɵ�������©���ݳ���
���AB��
��2������Ʒ��̼�������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 3.3g
$\frac{100}{x}$=$\frac{44}{3.3g}$��
x=7.5g��
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��$\frac{7.5g}{10g}$��100%=75%��
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ75%��
��3��A��yg-zg�Ƿ�Ӧ��ϡ�����������ٳ���10%�Ƿ�Ӧ���Ȼ��������������Ȼ��������ͷ�Ӧ�Ļ�ѧ����ʽ���Լ���̼�����������һ�����Լ���̼��������������÷������У�
B��������Һ����ˡ��������ᾧ����ɣ��Ƶù����Ȼ�������Ϊyg�������Ȼ��������ͷ�Ӧ�Ļ�ѧ����ʽ���Լ���̼�����������һ�����Լ���̼��������������÷������У�
C���ⶨ����Һ����ˮ������Ϊyg�����а���ϡ�������ܼ�ˮ�������ͷ�Ӧ���ɵ�ˮ������������ȷ����Ӧ����ˮ�����������ܼ���̼��Ƶ��������Ӷ����ܼ���̼��Ƶ������������÷��������У�
���AB��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1����֪�����صĻ�ѧʽΪC15H22O5��������˵����ȷ����B��
A�����������ڻ���� B��һ�������ط����к���42��ԭ��
C�������صĺ�̼��Ϊ28.4% D��������C��H��O����Ԫ�ص�������Ϊ15��22��5
��2��ij��ȤС���ͬѧ��ͬһ�ص�ɼ��ƻ���ĸ�������Ҷ��Ҷ��7�죩�����ɼ�3�Σ����ⶨ��Ʒ�������صĺ�������¼�������
| �����غ�����mg/g�� | ||||
| ��1�� | ��2�� | ��3�� | ƽ��ֵ | |
| �� | 1.02 | 1.05 | 1.14 | 1.07 |
| �� | 0.09 | 0.11 | 0.10 | 0.10 |
| Ҷ��Ҷ��7�죩 | 4.57 | 4.65 | 4.58 | 4.60 |
| A�� | һ����̼���� | |
| B�� | �ڿ��о�������ͷʱ�е�����ζ | |
| C�� | �øɱ�������������˹����� | |
| D�� | ʹ��ú����ȼ���շ����� |
| A�� | CO��CO2 ���ó���ʯ��ˮ���� | |
| B�� | CO���л�ԭ�ԣ�����������ұ�� | |
| C�� | CO2�Ĺ����ŷſɵ�������IJ��� | |
| D�� | ���ʯ��ʯī�ṹ��̼ԭ�����з�ʽ��ͬ |
 ȼ�������ǵ����������ķ�չ�������е���ϵ��
ȼ�������ǵ����������ķ�չ�������е���ϵ��