��Ŀ����
5��ijͬѧΪ̽��һƿ��ɫҺ��A�ijɷ֣���������ʵ�飺����A�м����ɫ��ĩB���ڳ����¾���Ѹ�ٲ�������C��ͬʱ�����˳�����ΪҺ��Ĵ�����D����B�������ͻ�ѧ�����ڷ�Ӧǰ��û�иı䣮
�ڸ�ͬѧ������C�ռ��������������ǵ�ľ������ʢ��C�ļ���ƿ�У�ľ������ȼ�գ�
��1������ݢ�д���������ʵ����ƣ�A�������⣬Dˮ��
��2��д��A��B�õ�C��D�����ֱ���ʽ����������$\stackrel{��������}{��}$ˮ+����������B������ã��˷�Ӧ���ڷֽ⣨����ϡ��ֽ⡱����Ӧ��
���� ��������ṩ����Ϣ���з��������A�м����ɫ��ĩB���ڳ����¾���Ѹ�ٲ�������C��ͬʱ�����˳�����ΪҺ��Ĵ�����D����B�������ͻ�ѧ�����ڷ�Ӧǰ��û�иı䣬�������ǵ�ľ������ʢ��C�ļ���ƿ�У�ľ������ȼ�գ���C��������B�������ͻ�ѧ�����ڷ�Ӧǰ��û�иı䣬��B�Ǵ��������������ڶ������̵Ĵ������·ֽ�����ˮ����������A�ǹ������⣬��D��ˮ��B�Ƕ������̣��ݴ˽��
��� �⣺��A�м����ɫ��ĩB���ڳ����¾���Ѹ�ٲ�������C��ͬʱ�����˳�����ΪҺ��Ĵ�����D����B�������ͻ�ѧ�����ڷ�Ӧǰ��û�иı佫�����ǵ�ľ������ʢ��C�ļ���ƿ�У�ľ������ȼ�գ���C��������A�ǹ������⣬�ڶ������̵Ĵ������·ֽ�����ˮ����������D��ˮ��B�Ƕ������̣�
��1��A�ǹ������⣬B�Ƕ������̣�D��ˮ������������⣬�������̣�ˮ��
��2�����������ڶ������̵Ĵ������·ֽ�����ˮ�������������������$\stackrel{��������}{��}$ˮ+�����������ֽ⣮
���� ���⿼����dz��������ʵ��ƶϣ���ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�

��ϰ��ϵ�д�
�����Ŀ
15��﮵�������͵ĸ��ܵ�أ��������ᡢ�������������ֻ���������Ե��û���������ij��﮵�ص��ܷ�Ӧ�ɱ�ʾLi+MnO2�TLiMnO2������˵����ȷ���ǣ�������
�ٸ÷�Ӧ��Mn�Ļ��ϼ۷����˱仯 �ڸ÷�Ӧ���ڻ��Ϸ�Ӧ
��LiMnO2Ϊ���͵������� ��LiMnO2Ϊ����
�ٸ÷�Ӧ��Mn�Ļ��ϼ۷����˱仯 �ڸ÷�Ӧ���ڻ��Ϸ�Ӧ
��LiMnO2Ϊ���͵������� ��LiMnO2Ϊ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
10��2016�ꡰ��������ա��������ǡ���Լ��Լ������Դ��������ɫ��Լ���������˵����ȷ���ǣ�������
| A�� | Ϊ���ũ�������������ʩ��ũҩ�ͻ��� | |
| B�� | ������Ⱦָ��Խ��������Խ�� | |
| C�� | ��ˮ�����롰�ೱ���շ��ĸ�Դ��ͬ | |
| D�� | ���á���ɫ��ѧ�����գ�ʹԭ�Ͼ�����ת��Ϊ����Ҫ������ |
17�����������ŷŵ������пɵ���������ǣ�������
| A�� | �������� | B�� | ������̼ | C�� | ���� | D�� | ���� |
14����ѧ����ʽ����Ҫ�Ļ�ѧ���ԣ����л�ѧ����ʽ��д��ȷ���ǣ�������
| A�� | H2O2$\frac{\underline{\;MnO_2\;}}{\;}$H2��+O2�� | B�� | 2Cu+O2=2CuO | ||
| C�� | C3H 8+5O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2��+4H2O | D�� | 3H2SO4+Fe2O3�TFe2��SO4��3+3H2O |
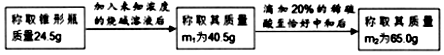
 ��ͥ�г��ý������Ч�ɷ������ᣬС��ͬѧ��ⶨijƷ�ƽ�����Ȼ�����������������Ƚ�һ�����Ľ����Ʒ�����ձ��У�Ȼ�������ձ��м���ʯ��ʯ��ʯ��ʯ�е����ʼȲ����ᷴӦ��Ҳ������ˮ���������ݲ���Ϊֹ����֪�ձ������ʵ�������ʯ��ʯ�������Ĺ�ϵͼ��ͼ��ʾ����
��ͥ�г��ý������Ч�ɷ������ᣬС��ͬѧ��ⶨijƷ�ƽ�����Ȼ�����������������Ƚ�һ�����Ľ����Ʒ�����ձ��У�Ȼ�������ձ��м���ʯ��ʯ��ʯ��ʯ�е����ʼȲ����ᷴӦ��Ҳ������ˮ���������ݲ���Ϊֹ����֪�ձ������ʵ�������ʯ��ʯ�������Ĺ�ϵͼ��ͼ��ʾ����