��Ŀ����
ijͬѧΪ�˲ⶨijʯ��ʯ��̼��Ƶ�����������ʯ��ʯ�е����ʲ������ᷴӦ��ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ���������±���
�Լ��㣺
��1�����������ڵ�1�β�õ������У� �������ʣ���ȫ��Ӧ��
��2��������m= g
��3�����ʯ��ʯ��Ʒ��̼��Ƶ�������������д��������̣�
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ��������g�� | 20 | 20 | 20 | 20 |
| ȡ�����������g�� | 50 | 100 | 150 | 200 |
| ��������������g�� | 2.2 | m | 6.6 | 6.6 |
��1�����������ڵ�1�β�õ������У�
��2��������m=
��3�����ʯ��ʯ��Ʒ��̼��Ƶ�������������д��������̣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����ݼ�¼���ݿɷ��֣���һ����������ʵ���У������������ɱ�������ʯ��ʯ��Ӧ�����������̼������Ҳ��Ӧ�ɱ����ӣ����ڵ�1�β�õ������У����ᣩ��ȫ��Ӧ��
��2�����ݼ�¼���ݿɷ��֣���һ����������ʵ���У������������ɱ�������ʯ��ʯ��Ӧ�����������̼������Ҳ��Ӧ�ɱ����ӣ��ҵ��Ĵ���������������������ɶ�����̼�����������֪��˵��������ʵ����ȫ��Ӧʱʯ��ʯ��ȫ��Ӧ���н��
��3�����ݶ�����̼�����������ʯ��ʯ��CaCO3�����������������ʯ��ʯ��CaCO3�������������ɣ�
��2�����ݼ�¼���ݿɷ��֣���һ����������ʵ���У������������ɱ�������ʯ��ʯ��Ӧ�����������̼������Ҳ��Ӧ�ɱ����ӣ��ҵ��Ĵ���������������������ɶ�����̼�����������֪��˵��������ʵ����ȫ��Ӧʱʯ��ʯ��ȫ��Ӧ���н��
��3�����ݶ�����̼�����������ʯ��ʯ��CaCO3�����������������ʯ��ʯ��CaCO3�������������ɣ�
����⣺��1�����ݼ�¼���ݿɷ��֣���һ����������ʵ���У������������ɱ�������ʯ��ʯ��Ӧ�����������̼������Ҳ��Ӧ�ɱ����ӣ����ڵ�1�β�õ������У����ᣩ��ȫ��Ӧ��
������
��2�����ݼ�¼���ݿɷ��֣���һ����������ʵ���У������������ɱ�������ʯ��ʯ��Ӧ�����������̼������Ҳ��Ӧ�ɱ����ӣ���m=4.4g
���4.4��
��3����ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 6.6g
=
x=15g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��
��100%=75%
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ75%��
������
��2�����ݼ�¼���ݿɷ��֣���һ����������ʵ���У������������ɱ�������ʯ��ʯ��Ӧ�����������̼������Ҳ��Ӧ�ɱ����ӣ���m=4.4g
���4.4��
��3����ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 6.6g
| 100 |
| x |
| 44 |
| 6.6g |
x=15g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��
| 15g |
| 20g |
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ75%��
������������Ҫ������ѧ������ͼ�����ݣ����������������Լ��ݷ���ʽ�����������

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ



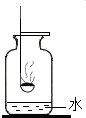 ijͬѧ�Խ̲�������������ȼ�յ�ʵ������˸Ľ�����������ǣ���ȼ�ճ��з�������ۣ���ȼ��Ѹ��������������ҵײ��н϶���ˮ�ļ���ƿ�У���ͼ��ʾ�����۲쵽ʵ�������������ȼ�ճ���ƿ��ˮ�У������ʵ����̣��ش��������⣺
ijͬѧ�Խ̲�������������ȼ�յ�ʵ������˸Ľ�����������ǣ���ȼ�ճ��з�������ۣ���ȼ��Ѹ��������������ҵײ��н϶���ˮ�ļ���ƿ�У���ͼ��ʾ�����۲쵽ʵ�������������ȼ�ճ���ƿ��ˮ�У������ʵ����̣��ش��������⣺