��Ŀ����
20��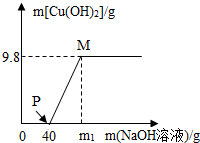 ��CuCl2��HCl��100g�����Һ�У���μ���������������Ϊ40%NaOH��Һ���μӷ�Ӧ��NaOH��Һ���������ɳ���������ϵ��ͼ����ͼ�ش�
��CuCl2��HCl��100g�����Һ�У���μ���������������Ϊ40%NaOH��Һ���μӷ�Ӧ��NaOH��Һ���������ɳ���������ϵ��ͼ����ͼ�ش���1��д��PM�η����Ļ�ѧ����ʽCuCl2+2NaOH�TCu��OH��2��+2NaCl��
��2��P����Һ�е�������NaCl CuCl2��
��3��ԭ�������CuCl2�������Ƕ��٣�
��4����ͨ������ȷ��m1��ֵ��
��5��M����Һ���������������Ƕ��٣����������0.1%��
���� ��1������NaOH��Һ������HCl��Ӧ������CuCl2��Ӧ������
��2������������Ӧ���̵������P��Ӧ�����жϣ�
��3�����ݳ�������������ԭ�������CuCl2��������
��4��m1��ֵ=40g+����9.8g������ͭ����������������Һ������
��5��M����Һ�е��������Ȼ��ƣ��Ȼ����������֣�һ�������������������ɵģ���һ�������Ȼ�ͭ���������Ʒ�Ӧ���ɵģ���Һ�������������غ㷨������Ļ����Һ��������+����������Һ������-���ɵ�������ͭ�������������Ӷ������Һ��������������
��� �⣺��1��CuCl2��HCl�Ļ����Һ�У���μ���NaOH��Һ���Ȼ��������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��Ȼ���Ȼ�ͭ�����������Ʒ�Ӧ����������ͭ�������Ȼ��ƣ���ѧ����ʽΪ��NaOH+HCl�TNaCl+H2O��CuCl2+2NaOH�TCu��OH��2��+2NaCl��
��2��P���ʾ�Ȼ������������ǡ����ȫ��Ӧ����ʱ�Ȼ�ͭ��û���������Ʒ�Ӧ������Һ�е�����ΪNaCl CuCl2��
��3��������9.8g������ͭ����������������Һ������Ϊx�������Ȼ��Ƶ�����Ϊz��CuCl2������w
CuCl2+2NaOH�TCu��OH��2��+2NaCl��
135 80 98 117
w x•40% 9.8g z
$\frac{135}{w}$=$\frac{80}{x•40%}$=$\frac{98}{9.8g}$=$\frac{117}{z}$
x=20g z=11.7g w=13.5g
��4���Ȼ�����������Ʒ�Ӧʱ���ĵ�����������Һ������Ϊ40g������m1=40g+20g=60g
��5�����Ȼ������������Ʒ�Ӧ�����Ȼ��Ƶ�����Ϊy
NaOH+HCl�TNaCl+H2O
40 58.5
40��40%=16g y
$\frac{40}{16g}$=$\frac{58.5}{y}$
��ã�y=23.4g
M����Һ�е������Ȼ��Ƶ�����Ϊ11.7g+23.4gg=35.1g
M����Һ��������������=$\frac{35.1g}{100g+60g-9.8g}$��100%=23.4%
��M����Һ��������������Ϊ23.4%
�ʴ�Ϊ����1��CuCl2+2NaOH�TCu��OH��2��+2NaCl����2��NaCl CuCl2��3��13.5g��4��60g��5��23.4%��
���� ���⿼�黯ѧ����ʽ����Һ���ۺϼ��㣬����ؼ��Ƿ��������Ӧ���̵��Ⱥ��ҳ�ÿ����������֪���ʵ�������

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�| A�� | ������Τ�����л��߷��ӻ����� | |
| B�� | ������Τ����Է�������Ϊ332 | |
| C�� | 1��������Τ������12��̼ԭ�ӡ�20����ԭ�ӡ�4����ԭ�Ӻ�7����ԭ�ӹ��� | |
| D�� | ������Τ��̼Ԫ�ء���Ԫ�غ͵�Ԫ�ص�������Ϊ3��5��1 |

| A�� | ��ʵ��֤ʵ���������������� | |
| B�� | ��ʵ�����̽��ˮ�����䲿λ�Ǿ���ľ�ʲ� | |
| C�� | �����������渲��һ���ɫ�����ʿ���˵�����Ľ�����Ա�ͭǿ | |
| D�� | ���е�����ģ��ʵ��֧�����������ʿ��ɷ���������ת�������Ŀ��� |
| A�� | ��˿�������о���ȼ��ʱ�������䣬�ų������ȣ��������������� | |
| B�� | �������ɫʯ���ˮ�д�������ɫʯ�����ɫ | |
| C�� | ����ˮ����ͭ��ĩ�еμ�����ˮ����ĩ����ɫ���ɫ | |
| D�� | �����ʯ��ˮ��ͨ�������̼����Һ����� |
 ��ҩ��������ɳ�ǣ�C3H8FN2O4���������������о�����ĺ�������Ⱦ����˵�����в���������ͼ��
��ҩ��������ɳ�ǣ�C3H8FN2O4���������������о�����ĺ�������Ⱦ����˵�����в���������ͼ��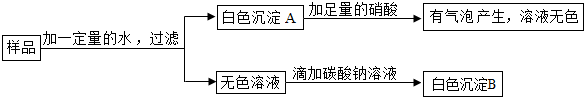

 ���ǵ���ʳס���뻯ѧ֪ʶ�ܲ��ɷ֣���������������е���ʵ��գ�
���ǵ���ʳס���뻯ѧ֪ʶ�ܲ��ɷ֣���������������е���ʵ��գ�