��Ŀ����
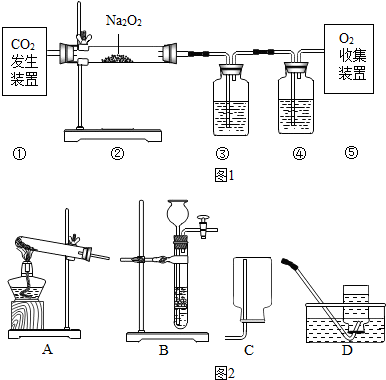
9�� ij��ѧ��ȤС���ͬѧ��ѧϰ�˿��������������IJⶨ�Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�
ij��ѧ��ȤС���ͬѧ��ѧϰ�˿��������������IJⶨ�Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£����������������װ�������ԣ�����װ�����������ã�
���ڼ���ƿ��װ������a�����ˮ��ȼ�ճ����һ�飬�����İ��ף����ھƾ����ϰѲ������¶˼��ȣ���ͼʾ�Ѽ���ƿ�еĿ����ܷ��������Ѽ���ƿ�ڵij��������쵽��ƿ�ף������ܵ���һ�˷���ʢ������ˮ���ձ��У�
�۰�Ԥ�ȼ��ȹ��IJ�������������Ӵ����۲쵽���������Ż�ȼ�ղ����������̣�ͬʱ���ȣ�
�ܴ�����ȼ��Ϩ����Լ���������ʧ��
�ݴ�����ƿ��ȫ��ȴ�����£��������ƿ��ˮ�������b����������ƿ�������c��
����a��b��c�������ݣ���������������������������ȼ�ճס����ס�������������������Բ��ƣ���
����������Ϣ���ش��������⣺
��1��д������ȼ�յ����ֱ���ʽ��+����$\stackrel{��ȼ}{��}$���������ף�ȼ�ճ��ڷ�����������ԭ����Ϊ�����ĵ�������
��2���ڰ���ʼȼ�յ����Ϩ��Ĺ����У�����ƿ��ˮ��ı仯��������½���������
��3����ʵ��ɹ�����д��a��b��c��������Ӧ�����һ����ʽ��ϵc+4a=5b��[b-a=$\frac{1}{5}$��c-a��]��
��4��С���ԱС����ʵ��ǰ������ƿ��Ҫװ������a�����ˮ���dz������⣬��ѯ��ʦ֮�����������ס�a�����ˮ�������ã��������������ɵġ����̡��⣬���мӿ췴Ӧ��ƿ���������ȴ�ٶȵ����ã�д��һ�㼴�ɣ���
��5��С���ԱС������䷢�ֲ���ݼ���ƿ�ڵ�ˮ�еμӼ���ʯ����Һ�����죮��һ����ʹ���ڳ�Ա�����ؿ�չ���ۣ�С����Ϊ�����ڶ�����̼����ˮ��ʹ��Һ�����ԣ���С�����Ϸ��ԣ������ж�����̼�ĺ������٣���ʵ������û�в���������̼���������ȷ�Ĺ۵㣬�Ա����������ĺ������룺���ǰ���ȼ�����ɵ���������������ˮ��������Һ�����ԣ���������������ˮ��Ӧ�����ᣩ��
���� ��1�����ݰ���ȼ�����������������
��2�����ݰ���ȼ��ʱƿ����������ı仯������н��ͣ�
��3����������Լռ������������֮һ�����ͼ�е������ϵ������ɣ�
��4��Χ�ơ�����ȼ��ʱ����ƿ��ѹ��������ȣ���ʹ����ƿ�������������Ͷ��ݳ�����һ���ؽ��з�����
��5����ʹʯ����Һ���ɫ����Һ�����Ե�֪ʶ���в��룮
��� �⣺��1������ȼ���������������ף�ȼ�յ����ֱ���ʽΪ����+����$\stackrel{��ȼ}{��}$���������ף�ȼ�ճ��ڷ�����������ԭ����Ϊ�����ĵ�������
��2������ȼ��ʱ��ų��������ȣ�����ƿ�ڿ�������������ͣ���ѹ����ʹ����ƿ�ڵ�ˮ�ز����������ձ��ڣ�ȼ��ֹͣ���¶��½���ƿ����ѹ��С���ձ��е�ˮ�ͻ����Ų����������뼯��ƿ�ڣ�������½���������
��3����������Լռ������������֮һ����ʵ��ɹ���a��b��c��������Ӧ�����һ����ʽ��ϵ�ǣ���b-a��=��c-a����$\frac{1}{5}$����c+4a=5b�����c+4a=5b��[b-a=$\frac{1}{5}$��c-a��]��
��4������ƿ��Ԥ��װ��������ˮ��ʹ����ȼ��ʱ����ƿ����ѹ���������������ʹ����ƿ�������������Ͷ��ݳ���
����ӿ췴Ӧ��ƿ���������ȴ�ٶȣ�
��5�������ǰ���ȼ�����ɵ���������������ˮ�����ԣ��μӼ�����ɫʯ����Һ�����죮
��������ǰ���ȼ�����ɵ���������������ˮ��������Һ�����ԣ���������������ˮ��Ӧ�����ᣩ��
�𰸣�
��1����+����$\stackrel{��ȼ}{��}$���������ף�Ϊ�����ĵ�������
��2�����½���������
��3��c+4a=5b��[b-a=$\frac{1}{5}$��c-a��]��
��4���ӿ췴Ӧ��ƿ���������ȴ�ٶȣ�
��5�����ǰ���ȼ�����ɵ���������������ˮ��������Һ�����ԣ���������������ˮ��Ӧ�����ᣩ��
���� ���������������IJⶨҲ���п������һ���ȵ㣬�ص㿼��ʵ������п����������Լ�����ʵ����ƫ���ƫС��ԭ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� |  ľ̿ | B�� |  ԭ�� | C�� |  ���ǡ����ǵ� | D�� |  �ɱ� |
| Ԫ������ | �� | ̼ | �� | �� | �� | �� |
| Ԫ�ط��� | H | C | O | Cl | Na | Fe |
��2����ij��ȼ������������Ԫ����ɣ�һ�����е�Ԫ����C��H�����ܺ��е�Ԫ����O��
��3�����ϱ��в���Ԫ�������һ��Һ��ȼ�ϣ��仯ѧʽΪ��C2H5OH��
��4����ijԪ�صĵ���A����������B����D��Һ��Ӧ���ֱ����ɸ�Ԫ�صĻ�����E��F��������Ԫ����B��F�еĻ��ϼ���ͬ��B��D��Һǡ����ȫ��Ӧ���������Fe2O3+6HCl=2FeCl3+3H2O��
��ҵ����F��H2��300-350��ʱ��Ӧ�Ƶ�D��E���÷�Ӧ�Ļ�ѧ����ʽΪFe2O3+3H2$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3H2O��

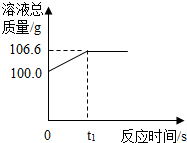 Ϊ�ⶨij������ʯ�����������������������ֺ�����ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��
Ϊ�ⶨij������ʯ�����������������������ֺ�����ͬѧ��������һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������һ����������������Һ��ȫ���գ�����Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ��