��Ŀ����
18��ijͬѧΪ�˲ⶨʵ�������������Ʒ�Ĵ��ȣ��������ʲ��μӷ�Ӧ�������������ȫ�ֽ⣩��ȡ2.5g����Ʒ��0.5g�������̻�ϣ����ȸû����t1ʱ�����ȴ������ʣ������������ظ����ϲ��������γƵü���t2��t3��t4ʱ���ʣ��������������¼���������| ����ʱ�� | t1 | t2 | t3 | t4 |
| ʣ���������� | 2.12 | 2.08 | 2.04 | 2.04 |
��2�����ȵ�t3ʱ���������Ƿ��Ѿ���ȫ��Ӧ���ǣ���ǡ�����
��3������ȫ��Ӧ��������������������ʵ�����
��4�������Ʒ������ص������ʹ��ȣ�
���� ������ڶ������̵Ĵ������£����ȷֽ������Ȼ��غ�������
��Ӧǰ��������Ϊ��Ӧ����������������
���ݷ�Ӧ�Ļ�ѧ����ʽ���ṩ�����ݿ��Խ�����ط���ļ��㣮
��� �⣺��1����Ӧ�Ļ�ѧ����ʽΪ��2KClO3$\frac{\underline{MnO_2}}{��}$2KCl+3O2����
��2�����ȵ�t3ʱ������������ټ�С��˵��������Ѿ���ȫ��Ӧ��
��3����ȫ��Ӧ��������������Ϊ��2.5g+0.5g-2.04g=0.96g��
0.96g���������ʵ���Ϊ��0.96g��32g/mol=0.03mol��
��4��������ص�����Ϊx��
2KClO3$\frac{\underline{MnO_2}}{��}$2KCl+3O2����
245 96
x 0.96g
$\frac{245}{x}$=$\frac{96}{0.96g}$��
x=2.45g��
����Ʒ������صĴ���Ϊ��$\frac{2.45g}{2.5g}$��100%=98%��
�ʴ�Ϊ��2KClO3$\frac{\underline{MnO_2}}{��}$2KCl+3O2�����ǣ�0.96g��0.03mol��2.45g��98%��
���� �������ڼ����е�Ӧ�úܹ㷺�����Ĺؼ���Ҫ���������ʵ���������Ҫ���δ֪��֮��Ĺ�ϵ���ٸ��ݾ����������⣮

��ϰ��ϵ�д�
�����Ŀ
8�����б仯�У����ڻ�ѧ�仯���ǣ�������
| A�� | �ƾ��ӷ� | B�� | ��ҩ��ը | C�� | ��ˮɹ�� | D�� | ��ʪ���·���� |
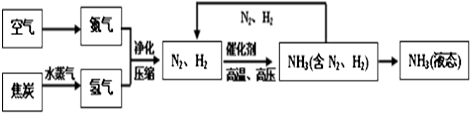

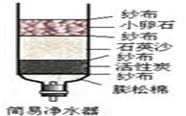 ˮ�����������в���ȱ�ٵ����ʣ�
ˮ�����������в���ȱ�ٵ����ʣ�