��Ŀ����
13��ʵ������һƿ���õ��������ƹ��壬ij��ȤС��ͬѧ���˽������������ס���ͬѧ����ȡ9.3g�������Ʒ�ֱ��������ʵ�飬ʵ��������£���1��ʵ��һ����ͬѧȡ��Ʒ����ˮʹ����ȫ�ܽ⣬Ȼ���������μ�����������Һ�����ó����������������������Һ������ϵ��ͼ1��ʾ����ش��������⣺
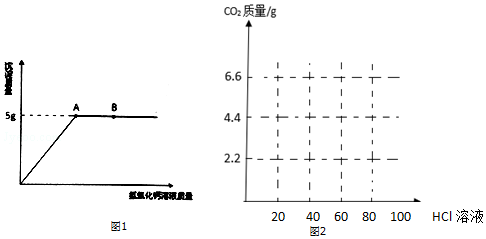
��B���Ӧ����Һ�е��������������ƺ��������ƣ�
��ʵ���������Ȼ�����Һ��������������Һ�����ó�������������5g������ڡ������ڡ���С�ڡ���
��2��������Ʒ��̼���Ƶ�������ͨ�������֪���������ƹ���ı��ʳ̶�Ϊ���ֱ��ʣ������ȫ���ʡ������ֱ��ʡ���û�б��ʡ���
��3��ʵ�������ͬѧȡ��Ʒ����ˮʹ����ȫ�ܽ⣬�����м�����������Ϊ7.3%��������Һ���μ���100��ʱ�����ٲ������壬����ͼ2�л����������������������Һ�����仯������ͼ��
���� ��1������B�����������ƹ����ĵ���з�����
��2�����ݳ����������ͻ�ѧ����ʽ���м���̼���Ƶ�������
��3����������������̼���Ƶ������Ƚ�����������������
��� �⣺��1����B���Ӧ����Һ���������ƺ�̼���Ʒ�Ӧ���������ƹ����ĵ㣬�����Һ�е������з�Ӧ���ɵ��������ƺ������������ƣ�����������ƺ��������ƣ�
��ʵ����������Ȼ�����Һ��������������Һ�����ó���̼��Ƶ�����ȡ����̼�����ϸ����ӵ����������������أ���������ȣ�������ڣ�
��2������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+CaCl2=CaCO3��+2NaCl
106 100
x 5g
$\frac{106}{x}=\frac{100}{5g}$
x=5.3g
��̼���Ƶ�����Ϊ5.3g��
��Ʒ������Ϊ9.3g����̼����Ϊ5.3g����˻������������ƣ����Dz��ֱ��ʣ�������ֱ��ʣ�
��3����Ʒ������Ϊ9.3g����̼����Ϊ5.3g��������������9.3g-5.3g=4.0g
�����������Ʒ�Ӧ������Ϊy����̼���Ʒ�Ӧ������Ϊz�����ɶ�����̼Ϊn
Na2CO3+2HCl=2NaCl+H2O+CO2����NaOH+HCl=NaCl+H2O
106 73 44 80 36.5
5.3g z��7.3% n 4.0g y��7.3%
$\frac{106}{5.3g}=\frac{73}{z��7.3%}$ $\frac{106}{5.3g}=\frac{44}{n}$ $\frac{80}{4.0g}=\frac{36.5g}{y��7.3%}$
z=50g��y=50g��n=2.2g
��ͼ�����������������Ʒ�Ӧ������̼���Ʒ�Ӧ������ͼʾΪ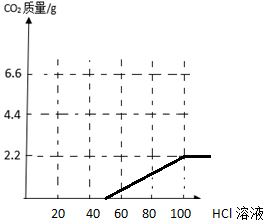 ��
��
���� �������й��������Ʊ��ʳɷֵ�̽���⣬�������и��ݷ���ʽ�ļ����⣬�ۺ��Խ�ǿ���ܹ�����ѧ���ķ�������������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | H2 | B�� | N2 | C�� | O2 | D�� | CO2 |
| A�� | �������� | B�� | Ʒ����ɢ | C�� | ����ȼ�� | D�� | �ɱ����� |
| A�� | ����ʯ�Ҹ����������� | |
| B�� | �ոѡ��Ӳݡ��������������з����Ƶü��� | |
| C�� | ��Ǧ���顢�������Ƴ���ºϽ� | |
| D�� | ��ɫֲ����CO2Ϊԭ�ϣ�����O2 |
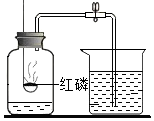 | 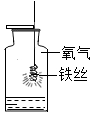 |  |  |
| A������Լռ���������$\frac{1}{5}$ | B����˿����������ȼ�� | C���ڢ۶Ա�˵������ȼ����Ҫ���� | D��������̼���ܶȱȿ�����ȼ��Ҳ��֧��ȼ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �մɲ�Ҷ�� | B�� | ������Ҷ�� | C�� | ��������Ҷ�� | D�� | ֽ�Ʋ�Ҷ�� |
 ��������Ϊ��ˮ
��������Ϊ��ˮ �¶�Խ�ߣ������˶�Խ����
�¶�Խ�ߣ������˶�Խ���� ��������Ҫ����
��������Ҫ���� ���������ǿ������������
���������ǿ������������