��Ŀ����
ij�о�С�����H2O2�ֽ�����H2O��O2��ʵ�����������о������ǽ���������ʵ�飺����ʢ��5mL 5% H2O2��Һ���Թ��У���������ǵ�ľ����ľ������ȼ������ʢ��5mL5%H2O2��Һ���Թ��У�����ag MnO2����������ǵ�ľ����ľ����ȼ������ʢ��5mL5%H2O2��Һ���Թ��У�����agFe2O3����������ǵ�ľ����ľ����ȼ���ܾ����飬�ڡ����з�Ӧ���Թ����Էֱ���ag MnO2��ag Fe2O3��
���⣺
��1��MnO2��Fe2O3��������Ӧ�е������� ��
��2��ʵ��ڡ�����H2O2��Ũ�ȣ�W%���� Ϊ�ˣ������������H2O2��Һ��ȡ��ͬ���O2�����ʱ�����±���
��3�����ϱ����ܵó���Щ���ۣ�A B ��
���⣺
��1��MnO2��Fe2O3��������Ӧ�е�������
��2��ʵ��ڡ�����H2O2��Ũ�ȣ�W%����
| Ũ��ʱ�䣨min������ | 30% H2O2 | 15% H2O2 | 5% H2O2 |
| ����ag MnO2 | 0.2 | 0.8 | 2.0 |
| ����ag Fe2O3 | 7.0 | 9.0 | 16.0 |
���㣺�������ص��������
ר�⣺��ѧ̽��
��������1���Т٢ڢ۶Աȿ�֪����������̡���������ʹ��Ӧ�ٶ�Ѹ����ߣ����ɢܿ�֪������û�иı䣬��˵�����Ǵ�����
��2��������֤�������Ĵ����ã���Ӧ�ô�����ͬ�������������б�Ҫ��
��3����ͼ�����ݿ��Կ�������������ͬʱ���Ƚϲ�ͬ�Ĵ�����������ͬʱ��֤����ҺŨ���뷴Ӧ�ٶȵĹ�ϵ��
��2��������֤�������Ĵ����ã���Ӧ�ô�����ͬ�������������б�Ҫ��
��3����ͼ�����ݿ��Կ�������������ͬʱ���Ƚϲ�ͬ�Ĵ�����������ͬʱ��֤����ҺŨ���뷴Ӧ�ٶȵĹ�ϵ��
����⣺��1���Т٢ڢ۶Աȿ�֪����������̡���������ķ�Ӧ�ٶ�������ߣ����ɢܿ�֪������û�иı䣬�ʿ�˵��������һ��Ӧ�Ĵ�����
�ʴ�Ϊ��������
��2���ڢ��Ǵ�����ͬ������ֻ�ı��������ʽ��жԱ�ʵ�飬�ڹ���������Һ�����������������������ͬ��������ʵ�飬���ܱȽϳ����Ӵ����ͼ���������̻���������Ĵ����ã�
�ʴ�Ϊ��5%
��3����ͼ�����ݿ��Կ�������������ͬʱ��������Ч����ͬ��������ͬʱ��֤����ҺŨ��Խ��Ӧ�ٶ�Խ��
�ʴ�Ϊ��A��ͬ�����£�MnO2�Ĵ�Ч����Fe2O3�ã�
B������������ͬ������£�H2O2Ũ��Խ�����������ٶ�Խ�죮
�ʴ�Ϊ��������
��2���ڢ��Ǵ�����ͬ������ֻ�ı��������ʽ��жԱ�ʵ�飬�ڹ���������Һ�����������������������ͬ��������ʵ�飬���ܱȽϳ����Ӵ����ͼ���������̻���������Ĵ����ã�
�ʴ�Ϊ��5%
��3����ͼ�����ݿ��Կ�������������ͬʱ��������Ч����ͬ��������ͬʱ��֤����ҺŨ��Խ��Ӧ�ٶ�Խ��
�ʴ�Ϊ��A��ͬ�����£�MnO2�Ĵ�Ч����Fe2O3�ã�
B������������ͬ������£�H2O2Ũ��Խ�����������ٶ�Խ�죮
���������⿼���˹���������ȡ����ʱ��ͬ������Ч�����Լ���Ӧ���Ũ���뷴Ӧ�ٶȵĹ�ϵ��Ҳ������ѧ������ͼ�����Ա�ʵ���������

��ϰ��ϵ�д�
�����Ŀ
����ʵ�����������ǣ�������
| A������Ƥ�������Թܿ�ʱ��Ӧ���Թܷ�����������������Ƥ�� |
| B�����������������Թ�ʱ��Ӧ�Ȱ��Թܺ�ţ��ѽ������������Թܿڣ��ٰ��Թ��������� |
| C��ϸ��ƿ��Һ��ʱ����ǩһ��Ҫ�������� |
| D�����������ʱ��Ӧ�Ȱѵ���һ�˷���ˮ�У���������ס�Թܣ��۲쵼�ܿ��Ƿ������ݲ��� |
��пƬͶ�뵽������Һ�һ��ʱ�����Һ�������ӣ���û������ų����ǣ�������
| A��ϡ���� | B��ϡ���� |
| C������þ��Һ | D������ͭ��Һ |
�й�ˮ�ĵ��ʵ�飬����˵����ȷ���ǣ�������
| A����������������������������1��2 |
| B��֤����ˮ����������������� |
| C�����Դ����������һ�˲������� |
| D��ˮ�ܵ��˵���˷����ǿɷֵ� |
һ�������£�76gCS2����96g������ǡ����ȫȼ�գ���ѧ��Ӧ����ʽ���£�CS2+3O2
CO2+2SO2����������������̼������Ԫ�ص��������ǣ�������
| ||
| A��1��2��3 |
| B��1��2��6 |
| C��3��16��12 |
| D��3��16��24 |
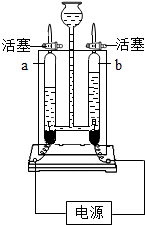 ��ͼ�ǵ��ˮ��ʵ��װ�ã���ͼ�ش�
��ͼ�ǵ��ˮ��ʵ��װ�ã���ͼ�ش�